Channel Behavior and Voltage Gating of a Cx43 Mutant Simulating Preconditioning
- PMID: 37746309
- PMCID: PMC10516231
- DOI: 10.1089/bioe.2023.0024
Channel Behavior and Voltage Gating of a Cx43 Mutant Simulating Preconditioning
Abstract
Background: Ischemic preconditioning induces lateralization and dephosphorylation of Connexin 43 (Cx43). However, the Cx43 protein that remains at intercalated disks may be phosphorylated by casein kinase 1 (CK1) and protein kinase C (PKC), and both kinases provide cardioprotection from further ischemic injury. Here we explore the channel characteristics of a Cx43 mutant mimicking preconditioning by CK1 and PKC phosphorylation.
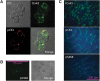
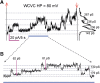
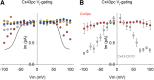
Materials and methods: Whole-cell patch-clamp recordings were performed in cells expressing the mutant Cx43pc (S325,328,330,368D, S365A-Cx43), and the connexin electrical behavior was analyzed at the single channel and macroscopic level.
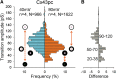
Results: Cx43pc hemichannels opened readily, whereas gap junctions channels displayed amplitudes between the wild-type and CK1 phosphorylated forms, and weaker voltage gating than either counterpart.
Conclusions: Ischemic preconditioning and the ensuing phosphorylation of Cx43 by PKC may render junctional channels insensitive to transjunctional voltages, allowing the preservation of intercellular communication in ischemic conditions.
Keywords: CK1; Cx43; PKC; ischemia; patch clamp; preconditioning.
Copyright 2023, Mary Ann Liebert, Inc., publishers.
Conflict of interest statement
No competing financial interests exist.
Figures



Similar articles
-
Cx43 Channel Gating and Permeation: Multiple Phosphorylation-Dependent Roles of the Carboxyl Terminus.Int J Mol Sci. 2018 Jun 4;19(6):1659. doi: 10.3390/ijms19061659. Int J Mol Sci. 2018. PMID: 29867029 Free PMC article.
-
Connexin 43 phosphorylation by casein kinase 1 is essential for the cardioprotection by ischemic preconditioning.Basic Res Cardiol. 2021 Mar 22;116(1):21. doi: 10.1007/s00395-021-00861-z. Basic Res Cardiol. 2021. PMID: 33751227 Free PMC article.
-
Functional formation of heterotypic gap junction channels by connexins-40 and -43.Channels (Austin). 2014;8(5):433-43. doi: 10.4161/19336950.2014.949188. Channels (Austin). 2014. PMID: 25483586 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Connexin 43 and ischemic preconditioning.Cardiovasc Res. 2004 May 1;62(2):335-44. doi: 10.1016/j.cardiores.2003.12.017. Cardiovasc Res. 2004. PMID: 15094353 Review.
References
-
- Cooper CD, Lampe PD. Casein kinase 1 regulates connexin-43 gap junction assembly. J Biol Chem 2002;277(47):44962–44968. - PubMed
-
- Beardslee MA, Lerner DL, Tadros PN, et al. . Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res 2000;87(8):656–662. - PubMed
-
- Srisakuldee W, Jeyaraman MM, Nickel BE, et al. . Phosphorylation of connexin-43 at serine 262 promotes a cardiac injury-resistant state. Cardiovasc Res 2009;83(4):672–681. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
