Ultrastructural and immunocytochemical evidence of a colonial nervous system in hydroids
- PMID: 37746552
- PMCID: PMC10512838
- DOI: 10.3389/fncir.2023.1235915
Ultrastructural and immunocytochemical evidence of a colonial nervous system in hydroids
Abstract
Background: As the sister group to all Bilateria, representatives of the phylum Cnidaria (sea anemones, corals, jellyfishes, and hydroids) possess a recognizable and well-developed nervous system and have attracted considerable attention over the years from neurobiologists and evo-devo researchers. Despite a long history of nervous system investigation in Cnidaria, most studies have been performed on unitary organisms. However, the majority of cnidarians are colonial (modular) organisms with unique and specific features of development and function. Nevertheless, data on the nervous system in colonial cnidarians are scarce. Within hydrozoans (Hydrozoa and Cnidaria), a structurally "simple" nervous system has been described for Hydra and zooids of several colonial species. A more complex organization of the nervous system, closely related to the animals' motile mode of life, has been shown for the medusa stage and a few siphonophores. Direct evidence of a colonial nervous system interconnecting zooids of a hydrozoan colony has been obtained only for two species, while it has been stated that in other studied species, the coenosarc lacks nerves.
Methods: In the present study, the presence of a nervous system in the coenosarc of three species of colonial hydroids - the athecate Clava multicornis, and thecate Dynamena pumila and Obelia longissima - was studied based on immunocytochemical and ultrastructural investigations.
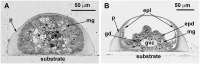
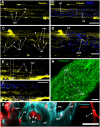
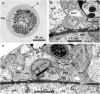
Results: Confocal scanning laser microscopy revealed a loose system composed of delicate, mostly bipolar, neurons visualized using a combination of anti-tyrosinated and anti-acetylated a-tubulin antibodies, as well as anti-RF-amide antibodies. Only ganglion nerve cells were observed. The neurites were found in the growing stolon tips close to the tip apex. Ultrastructural data confirmed the presence of neurons in the coenosarc epidermis of all the studied species. In the coenosarc, the neurons and their processes were found to settle on the mesoglea, and the muscle processes were found to overlay the nerve cells. Some of the neurites were found to run within the mesoglea.
Discussion: Based on the findings, the possible role of the colonial nervous system in sessile hydroids is discussed.
Keywords: Clava multicornis; Cnidaria; Dynamena pumila; Hydrozoa; Obelia longissima; coenosarc; colonial nervous system.
Copyright © 2023 Kosevich.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Hydroids (Cnidaria, Hydrozoa) from Mauritanian Coral Mounds.Zootaxa. 2020 Nov 16;4878(3):zootaxa.4878.3.2. doi: 10.11646/zootaxa.4878.3.2. Zootaxa. 2020. PMID: 33311142
-
Organizer regions in marine colonial hydrozoans.Zoology (Jena). 2015 Apr;118(2):89-101. doi: 10.1016/j.zool.2014.12.001. Epub 2015 Feb 7. Zoology (Jena). 2015. PMID: 25749284
-
Epithelial folding in the morphogenesis of the colonial marine hydrozoan, Dynamena pumila.Biosystems. 2018 Nov;173:157-164. doi: 10.1016/j.biosystems.2018.09.008. Epub 2018 Sep 21. Biosystems. 2018. PMID: 30248369
-
Biology and ecology of the hydrocoral millepora on coral reefs.Adv Mar Biol. 2006;50:1-55. doi: 10.1016/S0065-2881(05)50001-4. Adv Mar Biol. 2006. PMID: 16782450 Review.
-
Insight into the molecular and functional diversity of cnidarian neuropeptides.Int J Mol Sci. 2015 Jan 23;16(2):2610-25. doi: 10.3390/ijms16022610. Int J Mol Sci. 2015. PMID: 25625515 Free PMC article. Review.
References
-
- Aizenshtadt T. B., Polteva D. G. (1981). Origin of germ cells and early stages of oogenesis in marine hydroid polyp obelia. Ontogenez 12, 243–250.
-
- Anderson P. A. V. (2004). Cnidarian neurobiology: what does the future hold? Hydrobiologia 530, 107–116. 10.1007/s10750-004-2660-x - DOI
-
- Antsulevich A. E. (2015). Hydrozoa (Hydroids and Hydromedusae) of Russian seas. St. Petersburg: St. Petersburg University Press.
-
- Beloussov L. V. (1973). Growth and morphogenesis of some marine hydrozoa according to histological data and time-lapse studies. Publ. Seto Mar. Biol. Lab. 20, 315–366.
-
- Beloussov L. V. (1991). Basic morphogenetic processes in hydrozoa and their evolutionary implications: an exercise in rational taxonomy. Hydrobiologia, 216/217, 61–67. 10.1007/BF00026444 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

