Module 4-Deficient CCN2/Connective Tissue Growth Factor Attenuates the Progression of Renal Fibrosis via Suppression of Focal Adhesion Kinase Phosphorylation in Tubular Epithelial Cells
- PMID: 37746701
- PMCID: PMC10569360
- DOI: 10.1080/10985549.2023.2253130
Module 4-Deficient CCN2/Connective Tissue Growth Factor Attenuates the Progression of Renal Fibrosis via Suppression of Focal Adhesion Kinase Phosphorylation in Tubular Epithelial Cells
Abstract
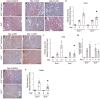
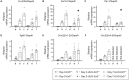
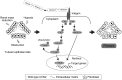
CCN2/connective tissue growth factor (CTGF) potentially serves as a therapeutic target for chronic kidney disease. Here we investigated CCN2 module-4, encoded by Ccn2 exon 5, through the generation of Ccn2 exon 5 knockout mice (Ex5-/- mice). To investigate renal fibrosis pathogenesis, Ex5-/- mice were employed to model unilateral ureteral obstruction (UUO), unilateral ischemic-reperfusion injury (UIRI), and 5/6 nephrectomy. Interstitial fibrosis was significantly attenuated in the Ex5-/- mice in the three models. Furthermore, phosphorylated focal adhesion kinase (FAK) levels in tubular epithelial cells were significantly lower in the kidneys of the UUO- and UIRI-Ex5-/- mice than those of the Ex5+/+ mice. Moreover, CCN2 module 4-mediated renal tubule FAK and promoted fibrosis. These findings indicate that CCN2 module-4-FAK pathway components will serve as therapeutic targets for effectively attenuating renal fibrosis.
Keywords: CCN2/CTGF; CKD; Chronic kidney disease; FAK; Focal adhesion kinase; centralized communication network 2; fibrosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA.. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2001;16:765–770. doi:10.1093/ndt/16.4.765. - DOI - PubMed
-
- Nagai Y, Matoba K, Kawanami D, Takeda Y, Akamine T, Ishizawa S, Kanazawa Y, Yokota T, Utsunomiya K, Nishimura R.. ROCK2 regulates TGF-beta-induced expression of CTGF and profibrotic genes via NF-kappaB and cytoskeleton dynamics in mesangial cells. Am J Physiol Renal Physiol. 2019;317:F839–F851. doi:10.1152/ajprenal.00596.2018. - DOI - PubMed
-
- Yokoi H, Mukoyama M, Sugawara A, Mori K, Nagae T, Makino H, Suganami T, Yahata K, Fujinaga Y, Tanaka I, et al. . Role of connective tissue growth factor in fibronectin expression and tubulointerstitial fibrosis. Am J Physiol Renal Physiol. 2002;282:F933–42. doi:10.1152/ajprenal.00122.2001. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
