Outcomes of Patients With Graves Disease 25 Years After Initiating Antithyroid Drug Therapy
- PMID: 37747433
- PMCID: PMC10876387
- DOI: 10.1210/clinem/dgad538
Outcomes of Patients With Graves Disease 25 Years After Initiating Antithyroid Drug Therapy
Abstract
Context: Graves disease (GD) is a leading cause of hyperthyroidism. Detailed investigations and predictors of long-term outcomes are missing.
Objective: This work aimed to investigate the outcomes in GD 25 years after initiating antithyroid drug treatment, including disease course, clinical and biochemical predictors of relapse, and quality of life.
Methods: A retrospective follow-up was conducted of GD patients that participated in a randomized trial from 1997 to 2001. Demographic and clinical data were obtained from medical records and questionnaires. Biobank samples were analyzed for inflammatory biomarkers and compared with age- and sex-matched healthy individuals.
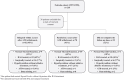
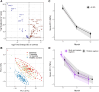
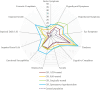
Results: We included 83% (182/218) of the patients from the original study. At the end of follow-up, normal thyroid function was achieved in 34%. The remaining had either active disease (1%), spontaneous hypothyroidism (13%), or had undergone ablative treatment with radioiodine (40%) or thyroidectomy (13%). Age younger than or equal to 40 years, thyroid eye disease (TED), smoking, and elevated levels of interleukin 6 and tumor necrosis factor receptor superfamily member 9 (TNFRS9) increased the risk of relapsing disease (odds ratio 3.22; 2.26; 2.21; 1.99; 2.36). At the end of treatment, CD40 was lower in patients who maintained normal thyroid function (P = .04). At the end of follow-up, 47% had one or more autoimmune diseases, including vitamin B12 deficiency (26%) and rheumatoid arthritis (5%). GD patients who developed hypothyroidism had reduced quality of life.
Conclusion: Careful lifelong monitoring is indicated to detect recurrence, hypothyroidism, and other autoimmune diseases. Long-term ATD treatment emerges as a beneficial first-line treatment option, especially in patients with young age at onset or presence of TED.
Keywords: Graves disease; autoimmunity; hypothyroidism; long-term follow up; quality of life; thyroid eye disease.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Excess mortality in treated and untreated hyperthyroidism is related to cumulative periods of low Serum TSH. J Clin Endocrinol Metab. 2017;102(7):2301‐2309. - PubMed
-
- Okosieme OE, Taylor PN, Evans C, et al. Primary therapy of Graves’ disease and cardiovascular morbidity and mortality: a linked-record cohort study. Lancet Diabetes Endocrinol. 2019;7(4):278‐287. - PubMed
-
- Törring O, Watt T, Sjölin G, et al. Impaired quality of life after radioiodine therapy compared to antithyroid drugs or surgical treatment for graves’ hyperthyroidism: A long-term follow-up with the thyroid-related patient-reported outcome questionnaire and 36-item short form health Status survey. Thyroid. 2019;29(3):322‐331. - PubMed
-
- Nedrebo BG, Holm PI, Uhlving S, et al. Predictors of outcome and comparison of different drug regimens for the prevention of relapse in patients with graves’ disease. Eur J Endocrinol. 2002;147(5):583‐589. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

