The effect of hypercapnia on the directional sensitivity of dynamic cerebral autoregulation and the influence of age and sex
- PMID: 37747437
- PMCID: PMC10993882
- DOI: 10.1177/0271678X231203475
The effect of hypercapnia on the directional sensitivity of dynamic cerebral autoregulation and the influence of age and sex
Abstract
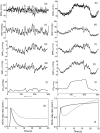
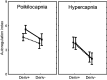
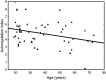
The cerebral circulation responds differently to increases in mean arterial pressure (MAP), compared to reductions in MAP. We tested the hypothesis that this directional sensitivity is reduced by hypercapnia. Retrospective analysis of 104 healthy subjects (46 male (44%), age range 19-74 years), with five minute recordings of middle cerebral blood velocity (MCAv, transcranial Doppler), non-invasive MAP (Finometer) and end-tidal CO2 (capnography) at rest, during both poikilocapnia and hypercapnia (5% CO2 breathing in air) produced MCAv step responses allowing estimation of the classical Autoregulation Index (ARIORIG), and corresponding values for both positive (ARI+D) and negative (ARI-D) changes in MAP. Hypercapnia led to marked reductions in ARIORIG, ARI+D and ARI-D (p < 0.0001, all cases). Females had a lower value of ARIORIG compared to males (p = 0.030) at poikilocapnia (4.44 ± 1.74 vs 4.74 ± 1.48) and hypercapnia (2.44 ± 1.93 vs 3.33 ± 1.61). The strength of directional sensitivity (ARI+D-ARI-D) was not influenced by hypercapnia (p = 0.46), sex (p = 0.76) or age (p = 0.61). During poikilocapnia, ARI+D decreased with age in females (p = 0.027), but not in males. Directional sensitivity was not affected by hypercapnia, suggesting that its origins are more likely to be inherent to the mechanics of vascular smooth muscle than to myogenic pathways.
Keywords: Cerebral blood flow; age; cerebral autoregulation; hypercapnia; sex.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Brassard P, Ferland-Dutil H, Smirl JDet al.. Evidence for hysteresis in the cerebral pressure-flow relationship in healthy men. Am J Physiol Heart Circ Physiol 2017; 312: H701–H704. - PubMed
-
- Numan T, Bain AR, Hoiland RLet al.. Static autoregulation in humans: a review and reanalysis. Med Eng Phys 2014; 36: 1487–1495. - PubMed
-
- Tzeng YC, Willie CK, Atkinson Get al.. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension 2010; 56: 268–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

