Current Understanding of Complement Proteins as Therapeutic Targets for the Treatment of Immunoglobulin A Nephropathy
- PMID: 37747686
- PMCID: PMC10807511
- DOI: 10.1007/s40265-023-01940-2
Current Understanding of Complement Proteins as Therapeutic Targets for the Treatment of Immunoglobulin A Nephropathy
Abstract
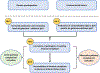
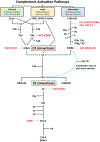
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis worldwide and a frequent cause of kidney failure. Currently, the diagnosis necessitates a kidney biopsy, with routine immunofluorescence microscopy revealing IgA as the dominant or co-dominant immunoglobulin in the glomerular immuno-deposits, often with IgG and sometimes IgM or both. Complement protein C3 is observed in most cases. IgAN leads to kidney failure in 20-40% of patients within 20 years of diagnosis and reduces average life expectancy by about 10 years. There is increasing clinical, biochemical, and genetic evidence that the complement system plays a paramount role in the pathogenesis of IgAN. The presence of C3 in the kidney immuno-deposits differentiates the diagnosis of IgAN from subclinical glomerular mesangial IgA deposition. Markers of complement activation via the lectin and alternative pathways in kidney-biopsy specimens are associated with disease activity and are predictive of poor outcome. Levels of select complement proteins in the circulation have also been assessed in patients with IgAN and found to be of prognostic value. Ongoing genetic studies have identified at least 30 loci associated with IgAN. Genes within some of these loci encode complement-system regulating proteins that can interact with immune complexes. The growing appreciation for the central role of complement components in IgAN pathogenesis highlighted these pathways as potential treatment targets and sparked great interest in pharmacological agents targeting the complement cascade for the treatment of IgAN, as evidenced by the plethora of ongoing clinical trials.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
JN is a co-founder and co-owner of and consultant for Reliant Glycosciences, LLC. JN is a co-inventor on US patent application 14/318,082 (assigned to UAB Research Foundation). JN has a sponsored research agreement with Travere Therapeutics. BAJ is a co-founder and co-owner of Reliant Glycosciences, LLC and a co-inventor on US patent application 14/318,082 (assigned to UAB Research Foundation). MBR is a co-founder and co-owner of and consultant for Reliant Glycosciences, LLC. MBR is a co-inventor on US patent application 14/318,082 (assigned to UAB Research Foundation). DVR received research funding from Reata Pharmaceuticals, Travere Therapeutics (Retrophin), Pfizer Pharmaceuticals, Calliditas Therapeutics (Pharmalink), Otsuka Pharmaceuticals (Visterra), Vertex Pharmaceuticals, Chinook Pharmaceuticals, consultancy fees from Novartis, GSK, George Clinical, Eledon Pharmaceuticals, Otsuka Pharmaceuticals (Visterra), Calliditas Therapeutics (Pharmalink), Chinook Pharmaceuticals. DVR is co-founder and co-owner of Reliant Glycosciences, LLC. None of the listed commercial entities contributed to this study. AR and TJG have nothing to disclose.
Figures

References
-
- Berger J, Hinglais N. intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris). 1968;74(9):694–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

