Dysfunction of CD169+ macrophages and blockage of erythrocyte maturation as a mechanism of anemia in Plasmodium yoelii infection
- PMID: 37748059
- PMCID: PMC10556621
- DOI: 10.1073/pnas.2311557120
Dysfunction of CD169+ macrophages and blockage of erythrocyte maturation as a mechanism of anemia in Plasmodium yoelii infection
Abstract
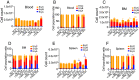
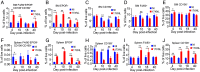
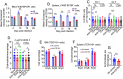
Plasmodium parasites cause malaria with disease outcomes ranging from mild illness to deadly complications such as severe malarial anemia (SMA), pulmonary edema, acute renal failure, and cerebral malaria. In young children, SMA often requires blood transfusion and is a major cause of hospitalization. Malaria parasite infection leads to the destruction of infected and noninfected erythrocytes as well as dyserythropoiesis; however, the mechanism of dyserythropoiesis accompanied by splenomegaly is not completely understood. Using Plasmodium yoelii yoelii 17XNL as a model, we show that both a defect in erythroblastic island (EBI) macrophages in supporting red blood cell (RBC) maturation and the destruction of reticulocytes/RBCs by the parasites contribute to SMA and splenomegaly. After malaria parasite infection, the destruction of both infected and noninfected RBCs stimulates extramedullary erythropoiesis in mice. The continuous decline of RBCs stimulates active erythropoiesis and drives the expansion of EBIs in the spleen, contributing to splenomegaly. Phagocytosis of malaria parasites by macrophages in the bone marrow and spleen may alter their functional properties and abilities to support erythropoiesis, including reduced expression of the adherence molecule CD169 and inability to support erythroblast differentiation, particularly RBC maturation in vitro and in vivo. Therefore, macrophage dysfunction is a key mechanism contributing to SMA. Mitigating and/or alleviating the inhibition of RBC maturation may provide a treatment strategy for SMA.
Keywords: erythropoiesis; malaria; mouse; phagocytosis; reticulocyte.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Plasmodium products contribute to severe malarial anemia by inhibiting erythropoietin-induced proliferation of erythroid precursors.J Infect Dis. 2014 Jan 1;209(1):140-9. doi: 10.1093/infdis/jit417. Epub 2013 Aug 6. J Infect Dis. 2014. PMID: 23922378
-
TLR7 modulates extramedullary splenic erythropoiesis in P. yoelii NSM-infected mice through the regulation of iron metabolism of macrophages with IFN-γ.Front Immunol. 2023 Apr 27;14:1123074. doi: 10.3389/fimmu.2023.1123074. eCollection 2023. Front Immunol. 2023. PMID: 37180169 Free PMC article.
-
Inhibition of Plasmodium yoelii blood-stage malaria by interferon alpha through the inhibition of the production of its target cell, the reticulocyte.Blood. 2001 Jun 15;97(12):3966-71. doi: 10.1182/blood.v97.12.3966. Blood. 2001. PMID: 11389041
-
Malaria, erythrocytic infection, and anemia.Hematology Am Soc Hematol Educ Program. 2009:87-93. doi: 10.1182/asheducation-2009.1.87. Hematology Am Soc Hematol Educ Program. 2009. PMID: 20008186 Free PMC article. Review.
-
Severe malarial anemia: innate immunity and pathogenesis.Int J Biol Sci. 2011;7(9):1427-42. doi: 10.7150/ijbs.7.1427. Epub 2011 Nov 2. Int J Biol Sci. 2011. PMID: 22110393 Free PMC article. Review.
Cited by
-
Erythroblastic island: the niche for erythroid terminal differentiation and beyond.Blood Sci. 2025 Mar 21;7(2):e00228. doi: 10.1097/BS9.0000000000000228. eCollection 2025 Jun. Blood Sci. 2025. PMID: 40129604 Free PMC article. Review.
-
Exploring CD169+ Macrophages as Key Targets for Vaccination and Therapeutic Interventions.Vaccines (Basel). 2025 Mar 20;13(3):330. doi: 10.3390/vaccines13030330. Vaccines (Basel). 2025. PMID: 40266235 Free PMC article. Review.
-
Malaria: Factors affecting disease severity, immune evasion mechanisms, and reversal of immune inhibition to enhance vaccine efficacy.PLoS Pathog. 2025 Jan 23;21(1):e1012853. doi: 10.1371/journal.ppat.1012853. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39847577 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

