Effect of Respiratory Viral Panel Adoption on Antibiotic Use in Ventilated Patients
- PMID: 37748086
- PMCID: PMC12039882
- DOI: 10.1513/AnnalsATS.202304-326OC
Effect of Respiratory Viral Panel Adoption on Antibiotic Use in Ventilated Patients
Abstract
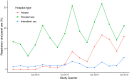
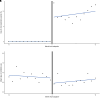
Rationale: Rapid respiratory viral panel (RVP) testing has become widely used to aid in the diagnosis and treatment of acute respiratory failure. However, the impact of RVP on antibiotic stewardship in critically ill patients is unclear. Objectives: To assess if adoption of RVP testing at hospitals was associated with changes in antibiotic duration in intensive care unit patients receiving invasive mechanical ventilation. Methods: With data from the Premier Inc. database from 2016 to 2019, we used interrupted time series with multivariable hierarchical linear regression models to quantify trends in outcomes for 31,644 patients in the 12 months before RVP adoption, the level change in outcomes at the time of RVP adoption (estimand of interest), and changes in outcome trends in the 12 months after RVP adoption. Results: Hospital adoption of RVP testing (n = 62,603) was associated with a decrease in days of antibiotics by 0.5 days (95% confidence interval, -0.8, -0.1) in the first month after adoption. There was also a significant decrease in the risk of Clostridioides difficile infection by 0.9% (95% confidence interval, -1.6, -0.3). There were no significant changes in other outcomes, including hospitalization costs, hospital length of stay, or rates of ventilator-associated pneumonia. Conclusions: Hospital adoption of RVP testing was associated with modest reductions in both antibiotic duration and risk of C. difficile infection among intensive care unit patients with acute respiratory failure and suspected infection.
Keywords: DNA probes; point-of-care testing; polymerase chain reaction; respiratory tract infections.
Figures
References
-
- Sakr Y, Ferrer R, Reinhart K, Beale R, Rhodes A, Moreno R, et al. IC-GLOSSARI Investigators; ESICM Trials Group The Intensive Care Global Study on Severe Acute Respiratory Infection (IC-GLOSSARI): a multicenter, multinational, 14-day inception cohort study. Intensive Care Med . 2016;42:817–828. - PMC - PubMed
-
- Brendish NJ, Malachira AK, Armstrong L, Houghton R, Aitken S, Nyimbili E, et al. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med . 2017;5:401–411. - PMC - PubMed
-
- Rogers BB, Shankar P, Jerris RC, Kotzbauer D, Anderson EJ, Watson JR, et al. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med . 2015;139:636–641. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

