Multiorgan MRI findings after hospitalisation with COVID-19 in the UK (C-MORE): a prospective, multicentre, observational cohort study
- PMID: 37748493
- PMCID: PMC7615263
- DOI: 10.1016/S2213-2600(23)00262-X
Multiorgan MRI findings after hospitalisation with COVID-19 in the UK (C-MORE): a prospective, multicentre, observational cohort study
Erratum in
-
Correction to Lancet Respir Med 2023; 11: 1003-19.Lancet Respir Med. 2023 Nov;11(11):e95. doi: 10.1016/S2213-2600(23)00386-7. Lancet Respir Med. 2023. PMID: 37914473 No abstract available.
Abstract
Introduction: The multiorgan impact of moderate to severe coronavirus infections in the post-acute phase is still poorly understood. We aimed to evaluate the excess burden of multiorgan abnormalities after hospitalisation with COVID-19, evaluate their determinants, and explore associations with patient-related outcome measures.
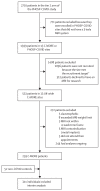
Methods: In a prospective, UK-wide, multicentre MRI follow-up study (C-MORE), adults (aged ≥18 years) discharged from hospital following COVID-19 who were included in Tier 2 of the Post-hospitalisation COVID-19 study (PHOSP-COVID) and contemporary controls with no evidence of previous COVID-19 (SARS-CoV-2 nucleocapsid antibody negative) underwent multiorgan MRI (lungs, heart, brain, liver, and kidneys) with quantitative and qualitative assessment of images and clinical adjudication when relevant. Individuals with end-stage renal failure or contraindications to MRI were excluded. Participants also underwent detailed recording of symptoms, and physiological and biochemical tests. The primary outcome was the excess burden of multiorgan abnormalities (two or more organs) relative to controls, with further adjustments for potential confounders. The C-MORE study is ongoing and is registered with ClinicalTrials.gov, NCT04510025.
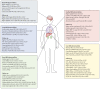
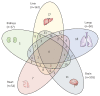
Findings: Of 2710 participants in Tier 2 of PHOSP-COVID, 531 were recruited across 13 UK-wide C-MORE sites. After exclusions, 259 C-MORE patients (mean age 57 years [SD 12]; 158 [61%] male and 101 [39%] female) who were discharged from hospital with PCR-confirmed or clinically diagnosed COVID-19 between March 1, 2020, and Nov 1, 2021, and 52 non-COVID-19 controls from the community (mean age 49 years [SD 14]; 30 [58%] male and 22 [42%] female) were included in the analysis. Patients were assessed at a median of 5·0 months (IQR 4·2-6·3) after hospital discharge. Compared with non-COVID-19 controls, patients were older, living with more obesity, and had more comorbidities. Multiorgan abnormalities on MRI were more frequent in patients than in controls (157 [61%] of 259 vs 14 [27%] of 52; p<0·0001) and independently associated with COVID-19 status (odds ratio [OR] 2·9 [95% CI 1·5-5·8]; padjusted=0·0023) after adjusting for relevant confounders. Compared with controls, patients were more likely to have MRI evidence of lung abnormalities (p=0·0001; parenchymal abnormalities), brain abnormalities (p<0·0001; more white matter hyperintensities and regional brain volume reduction), and kidney abnormalities (p=0·014; lower medullary T1 and loss of corticomedullary differentiation), whereas cardiac and liver MRI abnormalities were similar between patients and controls. Patients with multiorgan abnormalities were older (difference in mean age 7 years [95% CI 4-10]; mean age of 59·8 years [SD 11·7] with multiorgan abnormalities vs mean age of 52·8 years [11·9] without multiorgan abnormalities; p<0·0001), more likely to have three or more comorbidities (OR 2·47 [1·32-4·82]; padjusted=0·0059), and more likely to have a more severe acute infection (acute CRP >5mg/L, OR 3·55 [1·23-11·88]; padjusted=0·025) than those without multiorgan abnormalities. Presence of lung MRI abnormalities was associated with a two-fold higher risk of chest tightness, and multiorgan MRI abnormalities were associated with severe and very severe persistent physical and mental health impairment (PHOSP-COVID symptom clusters) after hospitalisation.
Interpretation: After hospitalisation for COVID-19, people are at risk of multiorgan abnormalities in the medium term. Our findings emphasise the need for proactive multidisciplinary care pathways, with the potential for imaging to guide surveillance frequency and therapeutic stratification.
Funding: UK Research and Innovation and National Institute for Health Research.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests BR received funding support from the British Heart Foundation (BHF) Oxford Centre of Research Excellence (CRE) and National Institute for Health and Care Research (NIHR) Oxford Biomedical Research Centre; and has received payment or honoraria for lectures, presentations, or educational events from Axcella Therapeutics. SN acknowledges grant support from the Oxford NIHR Biomedical Research Centre and the Oxford BHF CRE; and is a shareholder in Perspectum. PP has received grant funding from the NIHR (NIHR 132243) in the past 36 months. OMK has received partial funding from the Imperial NIHR Biomedical Research Centre; a grant from Cepheid; and speaker fees from Otsuka and Oxford Diagnostics. CEBo was supported by NIHR Nottingham Biomedical Research Centre. DGW received grant funding from NIHR; and payment or honoraria for lectures, presentations, or educational events from Biomerieux. TC has received grants on long COVID from NIHR and Guy's and Saint Thomas' Charity; carried out workshops on long COVID for NHS England and the European Association for Behavioural and Cognitive Therapies Conference; is an author of books related to fatigue and has received royalties in the past; and is the Director of the Persistent Physical Symptom Research and Treatment Service, which provides assessment and treatment to patients with long COVID. RA has received travel grant and lecture fees from Boehringer Ingelhem. ADS received grant funding from Bayer, Gilead, Pfizer, AstraZeneca, and Novartis; consulting fees from Boehringer Ingelheim, Bayer, Gilead, Pfizer, GSK, AstraZeneca, and Novartis; support for attending meetings or travel from AstraZeneca, Chiesi, and GSK; and has participated on a data safety monitoring board (DSMB) or advisory board for Bayer. LSH received fees for advisory boards or scientific steering committees from Janssen, Gossamer Bio, Bayer, Endotronix, Merck Sharp & Dohme, GSK, United Therapeutics, and LungRx; speaker fees from Janssen and Bayer; research support from Janssen; conference and travel support from Janssen; and has shareholdings in OneWelbeck Clinic, ATXA Therapeutics, iOWNA, and Circular. LGH received grant funding from GSK, Schering Plough, Synairgen, Novartis, Roche/Genentech, and MedImmune; consulting fees from AstraZeneca, Novartis, Roche/Genentech, Sanofi, Circassia, GSK, Chiesi, and Teva; support for attending meetings or travel from AstraZeneca, Chiesi, Novartis, Boehringer Ingelheim, Teva, and GSK; and has participated on a DSMB or advisory board for Novartis, Roche/Genentech, GSK, Evelo Biosciences, Teva, Theravance, and Vectura. GC received payment or honoraria for lectures, presentations, or educational events from GSK, Chiesi, AstraZeneca, and Boehringer Ingelheim; has participated on a DSMB or advisory board for AstraZeneca, acting as a chronic obstructive pulmonary disease group chair; and had a leadership role in the Scottish Government Respiratory Unscheduled Care Group and Lothian respiratory managed clinical network. DP received support for the present manuscript from the NIHR and UKRI. MGS has participated on a DSMB or advisory board for Pfizer; had a leadership or fiduciary role in Integrum Scientific and MedEx Solutions; has or had stocks or stock options in Integrum Scientific and MedEx Solutions; has received funding for equipment, material, or medical drugs from Chiesi; has been a non-remunerated independent member of the HMG UK Scientific Advisory Group for Emergencies, COVID-19 Response, and HMG UK New Emerging Respiratory Virus Threats Advisory Group; and received support for the present manuscript from the UKRI–MRC and Department of Health and Social Care NIHR. MGJ received grant funding from the Medical Research Council (MRC), British Lung Foundation, Royal Society, Boehringer Ingelheim, and the AAIR Charity. CMa was partly funded by the University College London (UCL) NIHR Biomedical Research Centre. SS is supported by grants from NIHR and Wellcome; and has received payment or honoraria for lectures, presentations, or educational events from GSK, Ministry of Justice, CIPLA, and Sherbourne Gibbs. SJS has participated on a DSMB or advisory board for the National Institute for Health and Care Excellence and on the Wales Long COVID advisory board; has or had a leadership or fiduciary role in the American Thoracic Society pulmonary rehabilitation assembly, Royal College of Physicians pulmonary rehabilitation accreditation scheme, and National Asthma and Chronic Obstructive Pulmonary Disease Audit Programme for pulmonary rehabilitation. SJS is also paid by the Royal College of Physicians for the audit and the Pulmonary Rehabilitation Services Accreditation Scheme. JKQ received grant funding from the MRC, HDR UK, GSK, Bayer, Boehringer Ingelheim, Asthma + Lung UK, Chiesi, and AstraZeneca; consulting fees from GSK and AstraZeneca; and payment or honoraria for lectures, presentations, or educational events from GSK, Boehringer Ingelheim, AstraZeneca, Chiesi, Teva, and Insmed. JDC received grant funding from AstraZeneca, Boehringer Ingelheim, Grifols, Insmed, Gilead Science, Genentech, and Novartis; and consulting fees from AstraZeneca, Boehringer Ingelheim, Janssen, Insmed, Chiesi, Grifols, Novartis, and Zambon. PJ received support for the present manuscript from the NIHR Oxford Biomedical Research Centre. MB and KM were supported by UKRI Project number 104688; National Consortium for Intelligent Medical Imaging. CAM was supported by an advanced Fellowship, NIHR301338, funded by the NIHR; acknowledges support from the University of Manchester BHF Accelerator Award (AA/18/4/34221) and the NIHR Manchester Biomedical Research Centre (NIHR203308); received support from the University of Manchester BHF Accelerator Award (AA/18/4/34221) and the NIHR Manchester Biomedical Research Centre (NIHR203308); received grant funding from NIHR Clinician scientist award, Roche Products, Univar Solutions, Amicus Therapeutics, Guerbet Laboratories, and Roche Diagnostics; received consulting fees from PureTech Health; received payment or honoraria for lectures, presentations, or educational events from Novo Nordisk and Boehringer Ingelheim; received support for attending meetings or travel from AstraZeneca; and has participated on a DSMB or advisory board for Boehringer Ingelheim and Lilly Alliance, AstraZeneca, and HAYA therapeutics. GJ received grant funding from AstraZeneca, Biogen, Galecto, GSK, Nordic Biosciences, RedX, Pliant; consulting fees from AstraZeneca, Brainomix, Bristol Myers Squibb, Chiesi, Cohbar, Daewoong, GSK, Veracyte, Resolution Therapeutics, and Pliant; payment or honoraria for lectures, presentations, or educational events from Boehringer Ingelheim, Chiesi, Roche, PatientMPower, and AstraZeneca; has participated on a DSMB or advisory board for Boehringer Ingelheim, Galapagos, and Vicore; and has or had a leadership or fiduciary role in NuMedii and Action for Pulmonary Fibrosis. VCH and AH received support for the present manuscript from the joint funding UKRI and NIHR (grant references: MR/V027859/1 and COV0319). AH reports funding support from NIHR Manchester Biomedical Research Centre; has received grants from the Engineering and Physical Sciences Research Council, Cystic Fibrosis Trust, NIHR, JP Moulton Charity, and Cystic Fibrosis Foundation; has received consulting fees from Roche Genentech and Vertex Pharmaceuticals; and has received payments for educational activities. SP is supported by a grant from BHF. JCP received support for the present manuscript from Breathing Matters, University College London Hospital Biomedical Research Centre NIHR; and has received payment or honoraria for lectures, presentations, or educational events from The Limbic. EMT holds a patent with Oxford University Innovation; and has or had stocks or stock options in Perspectum. She received grant funding from HDR UK BREATHE Hub; has participated on AstraZeneca's Thrombotic Thrombocytopenic Advisory Board; and had a leadership or fiduciary role in various UK and Scottish Government COVID-19 advisory bodies. MT has received consulting fees from Morphogen IX and Janssen; support for attending meetings or travel from GSK and Janssen; and has participated on a DSMB or advisory board for ComCov, FluCov. SRJ received grant funding from UKRI, European and Developing Countries Clinical Trials Partnership, MRC, Rosetrees Trust, and Global Challenges Research Fund; received payment or honoraria for lectures, presentations, or educational events from the Federation of European Academies of Medicine; has participated on a DSMB or advisory board for Bexero trial; and had a leadership or fiduciary role at the journal AIDS. VMF acknowledges support from the BHF (CH/16/1/32013). SKP acknowledges indirect support from the Oxford BHF CRE and NIHR Oxford Biomedical Research Centre. MK has or had stocks or stock options in Perspectum; and has financial or non-financial interests in Perspectum. CEBr received grant funding from GSK, AstraZeneca, Sanofi, Boehringer Ingelheim, Chiesi, Novartis, Roche, Genentech, Mologic, and 4D Pharma; and has received consulting fees from GSK, AstraZeneca, Sanofi, Boehringer Ingelheim, Chiesi, Novartis, Roche, Genentech, Mologic, 4D Pharma, and TEVA. In the past 3 years, GPM received grant funding from BHF and MRC; had a leadership or fiduciary role in the Wellcome Trust Early Career Committee and British Society of Cardiovascular Magnetic Resonance Research; and has received funding for equipment, material, or medical drugs from Circle Cvi42. RAE received grant funding from NIHR–UKRI–Wolfson Foundation; consulting fees from AstraZeneca for long COVID; payment or honoraria for lectures, presentations, or educational events from Boehringer Ingelheim; support for attending meetings or travel from Chiesi; and had a leadership or fiduciary role in the European Respiratory Society Pulmonary Rehabilitation Group. JRH received consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, and GSK; support for attending meetings or travel from AstraZeneca; and participated on a DSMB or advisory board for the study called HEAL COVID. LVW received support for the present manuscript from the UKRI, NIHR, and GSK and Asthma + Lung UK; grant funding from Orion Pharma, GSK, Genentech, and AstraZeneca; consulting fees from Galapagos and Boehringer Ingelheim; support for attending meetings or travel from Genentech; has participated on a DSMB or advisory board for Galapagos; and had a leadership role (Associate Editor) for the European Respiratory Journal. JJ received consulting fees from Boehringer Ingelheim, Roche, GSK, and NHSX; payment or honoraria for lectures, presentations, or educational events from Boehringer Ingelheim, Roche, GSK, and Takeda; support for attending meetings or travel from Boehringer Ingelheim; holds a patent with UK patent application number 2113765.8; and has participated on a DSMB or advisory board for Boehringer Ingelheim and Roche. PJMO is supported by a NIHR Senior Investigator Award (award 201385); has consulted for Janssen, Moderna, Cephid, Seqirus, Pfizer, GSK, and Sanofi in relation to vaccine development; and receives funding from EMINENT, a joint programme award from the MRC UK and GSK. All other members of the writing committee declare no competing interests.
Figures

Comment in
-
Making sense of multiorgan MRI imaging for post-acute sequelae of SARS-CoV-2 infection.Lancet Respir Med. 2023 Nov;11(11):951-952. doi: 10.1016/S2213-2600(23)00347-8. Epub 2023 Sep 22. Lancet Respir Med. 2023. PMID: 37748494 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

