Room temperature crystallography and X-ray spectroscopy of metalloenzymes
- PMID: 37748830
- PMCID: PMC10799221
- DOI: 10.1016/bs.mie.2023.07.009
Room temperature crystallography and X-ray spectroscopy of metalloenzymes
Abstract
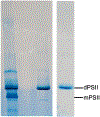
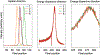
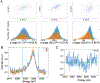
The ultrashort (10s of femtoseconds) X-ray pulses generated by X-ray free electron lasers enable the measurement of X-ray diffraction and spectroscopic data from radiation-sensitive metalloenzymes at room temperature while mostly avoiding the effects of radiation damage usually encountered when performing such experiments at synchrotron sources. Here we discuss an approach to measure both X-ray emission and X-ray crystallographic data at the same time from the same sample volume. The droplet-on-tape setup described allows for efficient sample use and the integration of different reaction triggering options in order to conduct time-resolved studies with limited sample amounts. The approach is illustrated by two examples, photosystem II that catalyzes the light-driven oxidation of water to oxygen, and isopenicillin N synthase, an enzyme that catalyzes the double ring cyclization of a tripeptide precursor into the β-lactam isopenicillin and can be activated by oxygen exposure. We describe the necessary steps to obtain microcrystals of both proteins as well as the operation procedure for the drop-on-tape setup and details of the data acquisition and processing involved in this experiment. At the end, we present how the combination of time-resolved X-ray emission spectra and diffraction data can be used to improve the knowledge about the enzyme reaction mechanism.
Keywords: Isopenicillin N synthase; Metalloenzymes; Photosystem II; Protein crystallography; X-ray emission spectroscopy; X-ray free electron laser.
Copyright © 2023. Published by Elsevier Inc.
Figures










Similar articles
-
X-ray Emission Spectroscopy as an in Situ Diagnostic Tool for X-ray Crystallography of Metalloproteins Using an X-ray Free-Electron Laser.Biochemistry. 2018 Aug 7;57(31):4629-4637. doi: 10.1021/acs.biochem.8b00325. Epub 2018 Jun 28. Biochemistry. 2018. PMID: 29906115 Free PMC article.
-
Sample efficient approaches in time-resolved X-ray serial crystallography and complementary X-ray emission spectroscopy using drop-on-demand tape-drive systems.Methods Enzymol. 2024;709:57-103. doi: 10.1016/bs.mie.2024.10.008. Epub 2024 Oct 23. Methods Enzymol. 2024. PMID: 39608948
-
Time-resolved studies of metalloproteins using X-ray free electron laser radiation at SACLA.Biochim Biophys Acta Gen Subj. 2020 Feb;1864(2):129466. doi: 10.1016/j.bbagen.2019.129466. Epub 2019 Oct 31. Biochim Biophys Acta Gen Subj. 2020. PMID: 31678142
-
Methods development for diffraction and spectroscopy studies of metalloenzymes at X-ray free-electron lasers.Philos Trans R Soc Lond B Biol Sci. 2014 Jul 17;369(1647):20130590. doi: 10.1098/rstb.2013.0590. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24914169 Free PMC article. Review.
-
Serial synchrotron and XFEL crystallography for studies of metalloprotein catalysis.Curr Opin Struct Biol. 2021 Dec;71:232-238. doi: 10.1016/j.sbi.2021.07.007. Epub 2021 Aug 26. Curr Opin Struct Biol. 2021. PMID: 34455163 Free PMC article. Review.
Cited by
-
X-ray Absorption Spectroscopy of Dilute Metalloenzymes at X-ray Free-Electron Lasers in a Shot-by-Shot Mode.J Phys Chem Lett. 2025 Apr 17;16(15):3778-3787. doi: 10.1021/acs.jpclett.5c00399. Epub 2025 Apr 7. J Phys Chem Lett. 2025. PMID: 40193717 Free PMC article.
References
-
- Agirre J, Atanasova M, Bagdonas H, Ballard CB, Baslé A, Beilsten-Edmands J, Borges RJ, Brown DG, Burgos-Mármol JJ, Berrisford JM, Bond PS, Caballero I, Catapano L, Chojnowski G, Cook AG, Cowtan KD, Croll TI, Debreczeni JÉ, Devenish NE, … Yamashita K (2023). The CCP4 suite: Integrative software for macromolecular crystallography. Acta Crystallographica Section D: Structural Biology, 79(6), Article 6. 10.1107/S2059798323003595 - DOI - PMC - PubMed

