Pretreatment Neurofilament Light Chain Serum Levels, Early Disease Severity, and Treatment Response in Pediatric Multiple Sclerosis
- PMID: 37748882
- PMCID: PMC10663003
- DOI: 10.1212/WNL.0000000000207791
Pretreatment Neurofilament Light Chain Serum Levels, Early Disease Severity, and Treatment Response in Pediatric Multiple Sclerosis
Erratum in
-
Corrections to Received Date Information.Neurology. 2024 Jul 9;103(1):e209596. doi: 10.1212/WNL.0000000000209596. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830175 Free PMC article. No abstract available.
Abstract
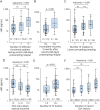
Background and objectives: High disease activity and frequent therapy failure in pediatric multiple sclerosis (MS) make prognostic biomarkers urgently needed. We investigated whether serum neurofilament light chain (sNfL) levels in treatment-naive pediatric patients with MS are associated with early disease severity and indicate treatment outcomes.
Methods: A retrospective cohort study of patients seen in the Göttingen Center for MS in Childhood and Adolescence, Germany. Inclusion criteria were MS diagnosis according to the McDonald criteria, MS onset <18 years, and available pretreatment serum sample. sNfL levels were analyzed using a single-molecule array assay. Associations with clinical and MRI evidence of disease severity at sampling were evaluated using the Spearman correlations and nonparametric tests for group comparisons. Correlations between pretreatment sNfL and annualized relapse and new T2 lesion rate on first-line therapy, and odd ratios for switch to high-efficacy therapy were assessed.
Results: A total of 178 patients (116 women [65%]) with a mean sampling age of 14.3 years were included in the study. Pretreatment sNfL levels were above the ≥90th percentile reported for healthy controls in 80% of patients (median 21.1 pg/mL) and correlated negatively with age, but no correlation was seen with sex, oligoclonal band status, or body mass index. High pretreatment sNfL levels correlated significantly with a high number of preceding relapses, a shorter first interattack interval, a high T2 lesion count, and recent gadolinium-enhancing lesions. Of interest, sNfL levels reflected more strongly MRI activity rather than clinical activity. Pretreatment sNfL levels also correlated significantly with the relapse rate and occurrence of new/enlarging T2 lesions while on first-line injectable therapy. Odds of future therapy escalation increased from 0.14 for sNfL below 7.5 pg/mL to 6.38 for sNfL above 15 pg/mL. In patients with a recent relapse, higher sNfL levels were associated with poorer recovery 3 months after attack.
Discussion: The results of this study have 3 important implications: First, pretreatment sNfL levels are a valuable biomarker for underlying disease activity in pediatric patients with MS. Second, pretreatment sNfL levels in pediatric patients with MS have a predictive value for the response to first-line therapy and the necessity of future therapy escalation. Third, high sNfL levels during a relapse are associated with poor recovery in this age group.
© 2023 American Academy of Neurology.
Conflict of interest statement
J. Gärtner, in the past 3 years, has received fees for lectures and consultancy fees from Bayer, Biogen, Merck, Novartis, and Sanofi. P. Huppke, in the past 3 years, has received speaker honoraria and consultancy fees from Biogen, Merck, Teva, and GW Pharma. All other authors report no relevant disclosures. Go to
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
