Movement Disorders in Patients With Genetic Developmental and Epileptic Encephalopathies
- PMID: 37748886
- PMCID: PMC10663013
- DOI: 10.1212/WNL.0000000000207808
Movement Disorders in Patients With Genetic Developmental and Epileptic Encephalopathies
Erratum in
-
Corrections to Received Date Information.Neurology. 2024 Jul 9;103(1):e209596. doi: 10.1212/WNL.0000000000209596. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830175 Free PMC article. No abstract available.
Abstract
Background and objectives: Movement disorders (MDs) are underrecognized in the developmental and epileptic encephalopathies (DEEs). There are now more than 800 genes implicated in causing the DEEs; relatively few of these rare genetic diseases are known to be associated with MDs. We identified patients with genetic DEEs who had MDs, classified the nature of their MDs, and asked whether specific patterns correlated with the underlying mechanism.
Methods: We classified the type of MDs associated with specific genetic DEEs in a large international cohort of patients and analyzed whether specific patterns of MDs reflected the underlying biological dysfunction.
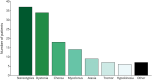
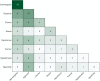
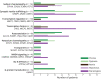
Results: Our cohort comprised 77 patients with a genetic DEE with a median age of 9 (range 1-38) years. Stereotypies (37/77, 48%) and dystonia (34/77, 44%) were the most frequent MDs, followed by chorea (18/77, 23%), myoclonus (14/77, 18%), ataxia (9/77, 12%), tremor (7/77, 9%), and hypokinesia (6/77, 8%). In 47% of patients, a combination of MDs was seen. The MDs were first observed at a median age of 18 months (range day 2-35 years). Dystonia was more likely to be observed in nonambulatory patients, while ataxia was less likely. In 46% of patients, therapy was initiated with medication (34/77, 44%), deep brain stimulation (1/77, 1%), or intrathecal baclofen (1/77, 1%). We found that patients with channelopathies or synaptic vesicle trafficking defects were more likely to experience dystonia; whereas, stereotypies were most frequent in individuals with transcriptional defects.
Discussion: MDs are often underrecognized in patients with genetic DEEs, but recognition is critical for the management of these complex neurologic diseases. Distinguishing MDs from epileptic seizures is important in tailoring patient treatment. Understanding which MDs occur with different biological mechanisms will inform early diagnosis and management.
© 2023 American Academy of Neurology.
Conflict of interest statement
S. van der Veen received funding from “Stichting Beatrixoord Noord-Nederland.” N. Specchio has served on scientific advisory boards for GW Pharma, BioMarin, Arvelle, Marinus, and Takeda, has received speaker honoraria from Eisai, Biomarin, Livanova, and Sanofi, and has served as an investigator for Zogenix, Marinus, Biomarin, UCB, and Roche. V.S.C. Fung receives a salary from NSW Health, has received unrestricted research grants from the Michael J. Fox Foundation, Abbvie, and Merz, has been on Advisory Boards for Abbvie, Allergan, Ipsen, Merz, Praxis, Seqirus, Stada, Teva, and UCB, and receives royalties from Health Press Ltd. I.E. Scheffer has served on scientific advisory boards for BioMarin, Chiesi, Eisai, Encoded Therapeutics, Knopp Biosciences, Takeda Pharmaceuticals, UCB, and Xenon Pharmaceuticals, has received speaker honoraria from UCB, BioMarin, Biocodex, Chiesi, Liva Nova, Zuellig Pharma, and Eisai, has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin, Encoded Therapeutics, and Eisai, has served as an investigator for Anavex Life Sciences, Cerecin Inc, Cerevel Therapeutics, Eisai, Encoded Therapeutics, EpiMinder Inc, Epygenyx, ES-Therapeutics, GW Pharma, Marinus, Neurocrine BioSciences, Ovid Therapeutics, Takeda Pharmaceuticals, UCB, Ultragenyx, Xenon Pharmaceuticals, Zogenix, and Zynerba, has consulted for Care Beyond Diagnosis, Epilepsy Consortium, Atheneum Partners, Ovid Therapeutics, UCB, Zynerba Pharmaceuticals, BioMarin, Encoded Therapeutics and Biohaven Pharmaceuticals, and is a Nonexecutive Director of Bellberry Ltd and a Director of the Australian Academy of Health and Medical Sciences and the Australian Council of Learned Academies Limited. She received support from the Australian Epilepsy Research Foundation grant, Australian National Health and Medical Research Council (NHMRC) Center for Research Excellence Grant (GNT2006841), Synergy Grant (GNT2010562), and Senior Investigator Fellowship (GNT1172897). She may accrue future revenue on pending patent WO61/010176 (filed: 2008): Therapeutic Compound, has a patent for SCN1A testing held by Bionomics Inc and licensed to various diagnostic companies, and has a patent molecular diagnostic/theranostic target for benign familial infantile epilepsy (BFIE) [PRRT2] 2011904493 & 2012900190 and PCT/AU2012/001321 (TECH ID: 2012-009). The remaining authors report no relevant discloses. Go to
Figures

Comment in
-
Recognition of Movement Disorders in Genetic, Developmental, and Epileptic Encephalopathies: More Than Seizures and Neurocognitive Problems.Neurology. 2023 Nov 7;101(19):815-816. doi: 10.1212/WNL.0000000000207973. Epub 2023 Sep 25. Neurology. 2023. PMID: 37748885 No abstract available.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
