Performance of a [18F]Flortaucipir PET Visual Read Method Across the Alzheimer Disease Continuum and in Dementia With Lewy Bodies
- PMID: 37748892
- PMCID: PMC10663007
- DOI: 10.1212/WNL.0000000000207794
Performance of a [18F]Flortaucipir PET Visual Read Method Across the Alzheimer Disease Continuum and in Dementia With Lewy Bodies
Erratum in
-
Corrections to Received Date Information.Neurology. 2024 Jul 9;103(1):e209596. doi: 10.1212/WNL.0000000000209596. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830175 Free PMC article. No abstract available.
Abstract
Background and objectives: Recently, the US Food and Drug Administration approved the tau-binding radiotracer [18F]flortaucipir and an accompanying visual read method to support the diagnostic process in cognitively impaired patients assessed for Alzheimer disease (AD). Studies evaluating this visual read method are limited. In this study, we evaluated the performance of the visual read method in participants along the AD continuum and dementia with Lewy bodies (DLB) by determining its reliability, accordance with semiquantitative analyses, and associations with clinically relevant variables.
Methods: We included participants who underwent tau-PET at Amsterdam University Medical Center. A subset underwent follow-up tau-PET. Two trained nuclear medicine physicians visually assessed all scans. Inter-reader agreement was calculated using Cohen κ. To examine the concordance of visual read tau positivity with semiquantification, we defined standardized uptake value ratio (SUVr) positivity using different threshold approaches. To evaluate the prognostic value of tau-PET visual read, we performed linear mixed models with longitudinal Mini-Mental State Examination (MMSE).
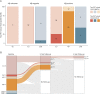
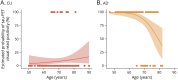
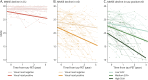
Results: We included 263 participants (mean age 68.5 years, 45.6% female), including 147 cognitively unimpaired (CU) participants, 97 amyloid-positive participants with mild cognitive impairment or AD dementia (AD), and 19 participants with DLB. The visual read inter-reader agreement was excellent (κ = 0.95, CI 0.91-0.99). None of the amyloid-negative CU participants (0/92 [0%]) and 1 amyloid-negative participant with DLB (1/12 [8.3%]) were tau-positive. Among amyloid-positive participants, 13 CU participants (13/52 [25.0%]), 85 with AD (85/97 [87.6%]), and 3 with DLB (3/7 [42.9%]) were tau-positive. Two-year follow-up visual read status was identical to baseline. Tau-PET visual read corresponded strongly to SUVr status, with up to 90.4% concordance. Visual read tau positivity was associated with a decline on the MMSE in CU participants (β = -0.52, CI -0.74 to -0.30, p < 0.001) and participants with AD (β = -0.30, CI -0.58 to -0.02, p = 0.04).
Discussion: The excellent inter-reader agreement, strong correspondence with SUVr, and longitudinal stability indicate that the visual read method is reliable and robust, supporting clinical application. Furthermore, visual read tau positivity was associated with prospective cognitive decline, highlighting its additional prognostic potential. Future studies in unselected cohorts are needed for a better generalizability to the clinical population.
Classification of evidence: This study provides Class II evidence that [18F]flortaucipir visual read accurately distinguishes patients with low tau-tracer binding from those with high tau-tracer binding and is associated with amyloid positivity and cognitive decline.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
E.M. Coomans, L.A. de Koning, R.M. Rikken, S.C.J. Verfaillie, D. Visser, A. den Braber, J. Tomassen, M. van de Beek, and S.S.V. Golla report no competing interests. L.E. Collij has received research support from GE Healthcare (paid to institution). A.W. Lemstra has been funded by ZonMW, Alzheimer Nederland, and Stichting Dioraphte. Research agreements with Combinostics and EPI Pharma. All funding is paid to her institution. A.D. Windhorst is Editor-in-Chief of
Figures


Comment in
-
From Clinical Trials to Memory Clinics, Tau-PET Visual Reads Can Help Diagnosis and Patient Stratification.Neurology. 2023 Nov 7;101(19):813-814. doi: 10.1212/WNL.0000000000207935. Epub 2023 Sep 25. Neurology. 2023. PMID: 37748880 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
