Haematopoietic stem cell transplantation for treatment of relapsing-remitting multiple sclerosis in Sweden: an observational cohort study
- PMID: 37748927
- PMCID: PMC10850659
- DOI: 10.1136/jnnp-2023-331864
Haematopoietic stem cell transplantation for treatment of relapsing-remitting multiple sclerosis in Sweden: an observational cohort study
Abstract
Background: A growing evidence base supports the use of autologous haematopoietic stem cell transplantation (aHSCT) for treatment of relapsing-remitting multiple sclerosis (RRMS), but it has not yet been integrated into most national clinical guidelines. The objective of this study was to assess efficacy and safety when aHSCT is implemented in routine healthcare.
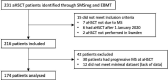
Methods: We assessed 231 patients and the final analysis included 174 RRMS patients who were treated with aHSCT in Sweden before 1 January 2020. Efficacy was evaluated by performing a retrospective analysis of prospectively collected data from the Swedish MS registry. Procedure-related safety was assessed by analysing data from electronic patient records covering a period of 100 days following aHSCT.
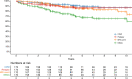
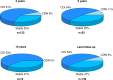
Results: With a median follow-up time of 5.5 (IQR: 3.4-7.5) years, the Kaplan-Meier estimate for no evidence of disease activity was 73% (95% CI 66% to 81%) at 5 years and 65% (95% CI 57% to 75%) at 10 years. Out of the 149 patients with baseline disability, 80 (54%) improved, 55 (37%) were stable and 14 (9%) deteriorated. The mean number of adverse events per patient was 1.7 (±SD: 1.5) for grade 3 events and 0.06 (±SD: 0.3) for grade 4 events. Febrile neutropenia was the most common adverse event, affecting 68% of patients. There was no treatment-related mortality.
Conclusions: Treatment with aHSCT for RRMS is associated with freedom from disease activity in a majority of patients, with acceptable adverse events. This procedure should be considered a standard of care for patients with highly active RRMS.
Keywords: CLINICAL NEUROLOGY; HAEMATOLOGY; MULTIPLE SCLEROSIS.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: EI has received speakers fee from Merck and honoraria from advisory boards for Sanofi-Aventis, Biogen and Merck. FP previously received research grants from Merck KGaA, Janssen and UCB outside this study. FP has received payment for expert testimony from Novartis. FP has participated in Data Monitoring Committee for clinical trials from Chugai, Lundbeck and Roche. JM has received lecture honorarium from Merck. NL has received honoraria from Sanofi. All other individual authors declare that there is no conflict of interest.
Figures