A comparison of machine learning methods to find clinical trials for inclusion in new systematic reviews from their PROSPERO registrations prior to searching and screening
- PMID: 37749068
- PMCID: PMC10872991
- DOI: 10.1002/jrsm.1672
A comparison of machine learning methods to find clinical trials for inclusion in new systematic reviews from their PROSPERO registrations prior to searching and screening
Abstract
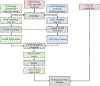
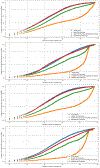
Searching for trials is a key task in systematic reviews and a focus of automation. Previous approaches required knowing examples of relevant trials in advance, and most methods are focused on published trial articles. To complement existing tools, we compared methods for finding relevant trial registrations given a International Prospective Register of Systematic Reviews (PROSPERO) entry and where no relevant trials have been screened for inclusion in advance. We compared SciBERT-based (extension of Bidirectional Encoder Representations from Transformers) PICO extraction, MetaMap, and term-based representations using an imperfect dataset mined from 3632 PROSPERO entries connected to a subset of 65,662 trial registrations and 65,834 trial articles known to be included in systematic reviews. Performance was measured by the median rank and recall by rank of trials that were eventually included in the published systematic reviews. When ranking trial registrations relative to PROSPERO entries, 296 trial registrations needed to be screened to identify half of the relevant trials, and the best performing approach used a basic term-based representation. When ranking trial articles relative to PROSPERO entries, 162 trial articles needed to be screened to identify half of the relevant trials, and the best-performing approach used a term-based representation. The results show that MetaMap and term-based representations outperformed approaches that included PICO extraction for this use case. The results suggest that when starting with a PROSPERO entry and where no trials have been screened for inclusion, automated methods can reduce workload, but additional processes are still needed to efficiently identify trial registrations or trial articles that meet the inclusion criteria of a systematic review.
Keywords: clinical trials; information retrieval; systematic reviews.
© 2023 The Authors. Research Synthesis Methods published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare that there is no potential conflict of interest.
Figures
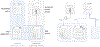

Similar articles
-
A shared latent space matrix factorisation method for recommending new trial evidence for systematic review updates.J Biomed Inform. 2018 Mar;79:32-40. doi: 10.1016/j.jbi.2018.01.008. Epub 2018 Feb 2. J Biomed Inform. 2018. PMID: 29410356
-
The automation of relevant trial registration screening for systematic review updates: an evaluation study on a large dataset of ClinicalTrials.gov registrations.BMC Med Res Methodol. 2021 Dec 18;21(1):281. doi: 10.1186/s12874-021-01485-6. BMC Med Res Methodol. 2021. PMID: 34922458 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Screening for the primary prevention of fragility fractures among adults aged 40 years and older in primary care: systematic reviews of the effects and acceptability of screening and treatment, and the accuracy of risk prediction tools.Syst Rev. 2023 Mar 21;12(1):51. doi: 10.1186/s13643-023-02181-w. Syst Rev. 2023. PMID: 36945065 Free PMC article.
-
Deep Neural Network for Reducing the Screening Workload in Systematic Reviews for Clinical Guidelines: Algorithm Validation Study.J Med Internet Res. 2020 Dec 30;22(12):e22422. doi: 10.2196/22422. J Med Internet Res. 2020. PMID: 33262102 Free PMC article.
References
-
- Pham B, Bagheri E, Rios P, Pourmasoumi A, Robson RC, Hwee J, et al. Improving the conduct of systematic reviews: a process mining perspective. Journal of Clinical Epidemiology. 2018. Nov;103:101–11. - PubMed
-
- Pieper D, Antoine SL, Neugebauer EAM, Eikermann M. Up-to-dateness of reviews is often neglected in overviews: a systematic review. Journal of Clinical Epidemiology. 2014. Dec;67(12):1302–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

