Blockade of interferon signaling decreases gut barrier integrity and promotes severe West Nile virus disease
- PMID: 37749080
- PMCID: PMC10520062
- DOI: 10.1038/s41467-023-41600-3
Blockade of interferon signaling decreases gut barrier integrity and promotes severe West Nile virus disease
Abstract
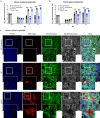
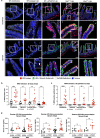
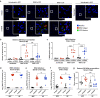
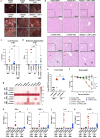
The determinants of severe disease caused by West Nile virus (WNV) and why only ~1% of individuals progress to encephalitis remain poorly understood. Here, we use human and mouse enteroids, and a mouse model of pathogenesis, to explore the capacity of WNV to directly infect gastrointestinal (GI) tract cells and contribute to disease severity. At baseline, WNV poorly infects human and mouse enteroid cultures and enterocytes in mice. However, when STAT1 or type I interferon (IFN) responses are absent, GI tract cells become infected, and this is associated with augmented GI tract and blood-brain barrier (BBB) permeability, accumulation of gut-derived molecules in the brain, and more severe WNV disease. The increased gut permeability requires TNF-α signaling, and is absent in WNV-infected IFN-deficient germ-free mice. To link these findings to human disease, we measured auto-antibodies against type I IFNs in serum from WNV-infected human cohorts. A greater frequency of auto- and neutralizing antibodies against IFN-α2 or IFN-ω is present in patients with severe WNV infection, whereas virtually no asymptomatic WNV-infected subjects have such antibodies (odds ratio 24 [95% confidence interval: 3.0 - 192.5; P = 0.003]). Overall, our experiments establish that blockade of type I IFN signaling extends WNV tropism to enterocytes, which correlates with increased gut and BBB permeability, and more severe disease.
© 2023. Springer Nature Limited.
Conflict of interest statement
M.S.D. is a consultant or advisor for Inbios, Vir Biotechnology, Senda Biosciences, Ocugen, Topspin Therapeutics, Moderna, and Immunome. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Vir Biotechnology, Emergent BioSolutions, and Moderna. J.-L.C. is an inventor on patent application PCT/US2021/042741 submitted by Rockefeller University that covers diagnosis of susceptibility to, and treatment of, viral disease and viral vaccines. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

