The road to approved vaccines for respiratory syncytial virus
- PMID: 37749081
- PMCID: PMC10519952
- DOI: 10.1038/s41541-023-00734-7
The road to approved vaccines for respiratory syncytial virus
Abstract
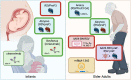
After decades of work, several interventions to prevent severe respiratory syncytial virus (RSV) disease in high-risk infant and older adult populations have finally been approved. There were many setbacks along the road to victory. In this review, I will discuss the impact of RSV on human health and how structure-based vaccine design set the stage for numerous RSV countermeasures to advance through late phase clinical evaluation. While there are still many RSV countermeasures in preclinical and early-stage clinical trials, this review will focus on products yielding long-awaited efficacy results. Finally, I will discuss some challenges and next steps needed to declare a global victory against RSV.
© 2023. Springer Nature Limited.
Conflict of interest statement
The author declares no competing interests.
Figures
References
-
- Blount RE, Jr., Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc. Soc. Exp. Biol. Med. 1956;92:544–549. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

