Rapid emergence and transmission of virulence-associated mutations in the oral poliovirus vaccine following vaccination campaigns
- PMID: 37749086
- PMCID: PMC10520055
- DOI: 10.1038/s41541-023-00740-9
Rapid emergence and transmission of virulence-associated mutations in the oral poliovirus vaccine following vaccination campaigns
Abstract
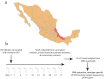
There is an increasing burden of circulating vaccine-derived polioviruses (cVDPVs) due to the continued use of oral poliovirus vaccine (OPV). However, the informativeness of routine OPV VP1 sequencing for the early identification of viruses carrying virulence-associated reversion mutations has not been directly evaluated in a controlled setting. We prospectively collected 15,331 stool samples to track OPV shedding from children receiving OPV and their contacts for ten weeks following an immunization campaign in Veracruz State, Mexico and sequenced VP1 genes from 358 samples. We found that OPV was genetically unstable and evolves at an approximately clocklike rate that varies across serotypes and by vaccination status. Overall, 61% (11/18) of OPV-1, 71% (34/48) OPV-2, and 96% (54/56) OPV-3 samples with available data had evidence of a reversion at the key 5' UTR attenuating position and 28% (13/47) of OPV-1, 12% (14/117) OPV-2, and 91% (157/173) OPV-3 of Sabin-like viruses had ≥1 known reversion mutations in the VP1 gene. Our results are consistent with previous work documenting rapid reversion to virulence of OPV and underscores the need for intensive surveillance following OPV use.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Rapid emergence and transmission of virulence-associated mutations in the oral poliovirus vaccine following vaccination campaigns.medRxiv [Preprint]. 2023 Mar 21:2023.03.16.23287381. doi: 10.1101/2023.03.16.23287381. medRxiv. 2023. Update in: NPJ Vaccines. 2023 Sep 25;8(1):137. doi: 10.1038/s41541-023-00740-9. PMID: 36993386 Free PMC article. Updated. Preprint.
Similar articles
-
Rapid emergence and transmission of virulence-associated mutations in the oral poliovirus vaccine following vaccination campaigns.medRxiv [Preprint]. 2023 Mar 21:2023.03.16.23287381. doi: 10.1101/2023.03.16.23287381. medRxiv. 2023. Update in: NPJ Vaccines. 2023 Sep 25;8(1):137. doi: 10.1038/s41541-023-00740-9. PMID: 36993386 Free PMC article. Updated. Preprint.
-
Sabin Vaccine Reversion in the Field: a Comprehensive Analysis of Sabin-Like Poliovirus Isolates in Nigeria.J Virol. 2015 Oct 14;90(1):317-31. doi: 10.1128/JVI.01532-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468545 Free PMC article.
-
Prevalence of vaccine-derived polioviruses in stools of immunodeficient children in South Africa.J Appl Microbiol. 2006 Dec;101(6):1367-79. doi: 10.1111/j.1365-2672.2006.03020.x. J Appl Microbiol. 2006. PMID: 17105568
-
Circulating vaccine-derived polioviruses: current state of knowledge.Bull World Health Organ. 2004 Jan;82(1):16-23. Epub 2004 Feb 26. Bull World Health Organ. 2004. PMID: 15106296 Free PMC article. Review.
-
Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative.Am J Epidemiol. 1999 Nov 15;150(10):1001-21. doi: 10.1093/oxfordjournals.aje.a009924. Am J Epidemiol. 1999. PMID: 10568615 Review.
Cited by
-
Is it time to switch to a formulation other than the live attenuated poliovirus vaccine to prevent poliomyelitis?Front Public Health. 2024 Jan 8;11:1284337. doi: 10.3389/fpubh.2023.1284337. eCollection 2023. Front Public Health. 2024. PMID: 38259741 Free PMC article. Review.
-
Oral live attenuated polio vaccines induce enhanced T-cell responses with broad antigen recognition compared to inactivated polio vaccines.medRxiv [Preprint]. 2025 May 21:2025.05.20.25328004. doi: 10.1101/2025.05.20.25328004. medRxiv. 2025. PMID: 40661308 Free PMC article. Preprint.
-
Degradation of Poliovirus Sabin 2 Genome After Electron Beam Irradiation.Vaccines (Basel). 2025 Jul 31;13(8):824. doi: 10.3390/vaccines13080824. Vaccines (Basel). 2025. PMID: 40872910 Free PMC article.
-
Elegant and Innovative Recoding Strategies for Advancing Vaccine Development.Vaccines (Basel). 2025 Jan 16;13(1):78. doi: 10.3390/vaccines13010078. Vaccines (Basel). 2025. PMID: 39852857 Free PMC article.
-
Live attenuated SARS-CoV-2 vaccine OTS-228 demonstrates efficacy, safety, and stability in preclinical model.NPJ Vaccines. 2025 May 23;10(1):104. doi: 10.1038/s41541-025-01165-2. NPJ Vaccines. 2025. PMID: 40404620 Free PMC article.
References
-
- Macklin, G. R. et al. Epidemiology of type 2 vaccine-derived poliovirus outbreaks between 2016 and 2020. Vaccine (2022) 10.1016/j.vaccine.2022.08.008. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

