Immunomodulatory effects of nanoparticles on dendritic cells in a model of allergic contact dermatitis: importance of PD-L2 expression
- PMID: 37749142
- PMCID: PMC10520013
- DOI: 10.1038/s41598-023-42797-5
Immunomodulatory effects of nanoparticles on dendritic cells in a model of allergic contact dermatitis: importance of PD-L2 expression
Abstract
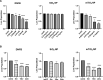
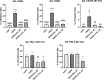
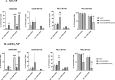
Nanoparticle (NP) skin exposure is linked to an increased prevalence of allergic contact dermatitis. In our prior studies using the mouse contact hypersensitivity (CHS) model, we reported that silica 20 nm (SiO2) NPs suppressed the allergic response and titanium dioxide NPs doped with manganese (mTiO2) exacerbated it. In this work, we conducted in vitro experiments using bone marrow-derived dendritic cells (BMDCs) to study the combinatorial effect of the potent 2,4-dinitrofluorobenzene (DNFB) hapten sensitizer with SiO2 and mTiO2 NPs on BMDC cytotoxicity, cytokine secretion and phenotype using the B7 family ligands. Results show that DNFB and mTiO2 behave similarly and exhibit proinflammatory characteristics while SiO2 promotes a naive phenotype. We observe that the B7-H3 (CD276) ligand is only expressed on CD80 + (B7-1) BMDCs. Results from adoptive transfer CHS studies, combined with BMDC phenotype analysis, point to the importance of PD-L2 expression in modulating the adaptive immune response. This work identifies metrics that can be used to predict the effects of NPs on contact allergy and to guide efforts to engineer cell-based therapies to induce hapten specific immune tolerance.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
Immunomodulatory Effects of Nanoparticles on Dendritic Cells in a Model of Allergic Contact Dermatitis - Importance of PD-L2 Expression.Res Sq [Preprint]. 2023 Jul 12:rs.3.rs-3069059. doi: 10.21203/rs.3.rs-3069059/v1. Res Sq. 2023. Update in: Sci Rep. 2023 Sep 25;13(1):15992. doi: 10.1038/s41598-023-42797-5. PMID: 37503107 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

