Incidence and molecular epidemiology of hepatitis C virus reinfection in prisons in Catalonia, Spain (Re-HCV study)
- PMID: 37749145
- PMCID: PMC10520040
- DOI: 10.1038/s41598-023-42701-1
Incidence and molecular epidemiology of hepatitis C virus reinfection in prisons in Catalonia, Spain (Re-HCV study)
Erratum in
-
Author Correction: Incidence and molecular epidemiology of hepatitis C virus reinfection in prisons in Catalonia, Spain (Re-HCV study).Sci Rep. 2023 Oct 12;13(1):17287. doi: 10.1038/s41598-023-44581-x. Sci Rep. 2023. PMID: 37828072 Free PMC article. No abstract available.
Abstract
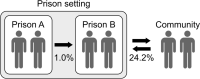
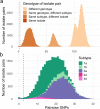
Hepatitis C virus (HCV) reinfection may hamper HCV elimination in prisons. We aimed to (i) determine the reinfection rate in people treated for HCV in Catalan prisons, (ii) measure reinfection in people entering prisons, and (iii) characterize the molecular epidemiology of HCV in prisons and people who inject drugs (PWID) in the community. Re-HCV was a prospective study in eight prisons (2019-2020) including two groups: (1) people cured with treatment in prison and followed-up every 6 months, and (2) people testing HCV-RNA positive at incarceration. Bio-behavioral data were collected. HCV isolates were sequenced and phylogenetically analyzed with those of PWID in the community. Reinfection follow-up after treatment was achieved in 97 individuals (103.05 person-years). Two reinfections were detected, resulting in an incidence ≤ 10/100 person-years. Among people entering prison, 2% (359/17,732) were viremic, of which 334 (93.0%) were included, and 44 (13.5%) presented with reinfection (84.7% being PWID). Frequently, HCV isolates in prisons and PWID in the community were phylogenetically related. Although HCV reinfection is low after treatment, it is common in people entering Catalan prisons. To maintain a low HCV prevalence in prisons, harm-reduction services and test-and-treat programs for PWID should be strengthened both inside and outside prisons.
© 2023. Springer Nature Limited.
Conflict of interest statement
Regarding competing interests, VS received travel sponsorship to attend scientific meetings from Gilead Sciences and Cepheid; AM presented lectures at Symposia or Conferences organized by Gilead, Janssen, MSD, Abbvie, and ViiV, as well as consulting services for MSD and Camurus; and EM received lecture fees and research grants from Abbott GmbH & Co. K. G, Gilead Sciences, Cepheid, and Abbvie. The other authors declare no competing interests.
Figures
References
-
- World Health Organization. Interim guidance for country validation of viral hepatitis elimination. Geneva: World Health Organization. https://www.who.int/publications-detail-redirect/9789240028395 (2021).
-
- Generalitat de Catalunya. Departament de Salut. Plà de prevenció i control de l’hepatitis C a Catalunya. http://salutpublica.gencat.cat/web/.content/minisite/aspcat/vigilancia_s... (2018).
-
- Ministerio de Sanidad Servicios Sociales e Igualdad. Strategic Plan for Tackling Hepatitis C in the Spanish National Health System 2015. https://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/hepatit... (2015).
-
- Marco, A., Guerrero, R. A. & Turu, E. Control of HCV infection in prisons in Catalonia, Spain. Compendium of good practices in the health sector response to viral hepatitis in the WHO European Region. Copenhagen: WHO Regional Office for Europe (2020).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

