The interface of condensates of the hnRNPA1 low-complexity domain promotes formation of amyloid fibrils
- PMID: 37749234
- PMCID: PMC10533390
- DOI: 10.1038/s41557-023-01289-9
The interface of condensates of the hnRNPA1 low-complexity domain promotes formation of amyloid fibrils
Abstract
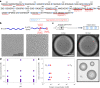
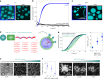
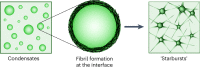
The maturation of liquid-like protein condensates into amyloid fibrils has been associated with several neurodegenerative diseases. However, the molecular mechanisms underlying this liquid-to-solid transition have remained largely unclear. Here we analyse the amyloid formation mediated by condensation of the low-complexity domain of hnRNPA1, a protein involved in amyotrophic lateral sclerosis. We show that phase separation and fibrillization are connected but distinct processes that are modulated by different regions of the protein sequence. By monitoring the spatial and temporal evolution of amyloid formation we demonstrate that the formation of fibrils does not occur homogeneously inside the droplets but is promoted at the interface of the condensates. We further show that coating the interface of the droplets with surfactant molecules inhibits fibril formation. Our results reveal that the interface of biomolecular condensates of hnRNPA1 promotes fibril formation, therefore suggesting interfaces as a potential novel therapeutic target against the formation of aberrant amyloids mediated by condensation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
RNA modulates hnRNPA1A amyloid formation mediated by biomolecular condensates.Nat Chem. 2024 Jul;16(7):1052-1061. doi: 10.1038/s41557-024-01467-3. Epub 2024 Mar 12. Nat Chem. 2024. PMID: 38472406 Free PMC article.
-
Decoupling Phase Separation and Fibrillization Preserves Activity of Biomolecular Condensates.Res Sq [Preprint]. 2025 Apr 29:rs.3.rs-6405673. doi: 10.21203/rs.3.rs-6405673/v1. Res Sq. 2025. PMID: 40343340 Free PMC article. Preprint.
-
Decoupling Phase Separation and Fibrillization Preserves Activity of Biomolecular Condensates.bioRxiv [Preprint]. 2025 Mar 19:2025.03.18.643977. doi: 10.1101/2025.03.18.643977. bioRxiv. 2025. PMID: 40166274 Free PMC article. Preprint.
-
Molecular structure in biomolecular condensates.Curr Opin Struct Biol. 2020 Feb;60:17-26. doi: 10.1016/j.sbi.2019.09.007. Epub 2019 Nov 29. Curr Opin Struct Biol. 2020. PMID: 31790873 Free PMC article. Review.
-
Liquid-liquid phase transitions and amyloid aggregation in proteins related to cancer and neurodegenerative diseases.Adv Protein Chem Struct Biol. 2019;118:289-331. doi: 10.1016/bs.apcsb.2019.08.002. Epub 2019 Sep 21. Adv Protein Chem Struct Biol. 2019. PMID: 31928729 Review.
Cited by
-
Biomolecular condensates can enhance pathological RNA clustering.Res Sq [Preprint]. 2024 Jul 19:rs.3.rs-4557520. doi: 10.21203/rs.3.rs-4557520/v1. Res Sq. 2024. Update in: Nat Chem. 2025 Aug;17(8):1236-1246. doi: 10.1038/s41557-025-01847-3. PMID: 39070659 Free PMC article. Updated. Preprint.
-
Accelerated amyloid fibril formation at the interface of liquid-liquid phase-separated droplets by depletion interactions.Protein Sci. 2025 Feb;34(2):e5163. doi: 10.1002/pro.5163. Protein Sci. 2025. PMID: 39876094 Free PMC article.
-
Thermophilic Behavior of Heat-Dissociative Coacervate Droplets.Nano Lett. 2024 Dec 18;24(50):15964-15972. doi: 10.1021/acs.nanolett.4c03058. Epub 2024 Nov 21. Nano Lett. 2024. PMID: 39573916 Free PMC article.
-
Silk fibroin as a surfactant for water-based nanofabrication.Nat Nanotechnol. 2024 Oct;19(10):1514-1520. doi: 10.1038/s41565-024-01720-3. Epub 2024 Jul 29. Nat Nanotechnol. 2024. PMID: 39075291
-
The mechanobiology of biomolecular condensates.Biophys Rev (Melville). 2025 Mar 25;6(1):011310. doi: 10.1063/5.0236610. eCollection 2025 Mar. Biophys Rev (Melville). 2025. PMID: 40160200 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

