New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders
- PMID: 37749255
- PMCID: PMC10520063
- DOI: 10.1038/s41598-023-43354-w
New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders
Erratum in
-
Author Correction: New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders.Sci Rep. 2023 Nov 8;13(1):19411. doi: 10.1038/s41598-023-45727-7. Sci Rep. 2023. PMID: 37938274 Free PMC article. No abstract available.
-
Author Correction: New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders.Sci Rep. 2024 Feb 6;14(1):3027. doi: 10.1038/s41598-024-53555-6. Sci Rep. 2024. PMID: 38321151 Free PMC article. No abstract available.
Abstract
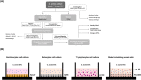
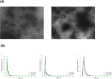
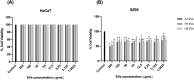
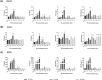
Cutibacterium acnes (C. acnes) is one of the most prevalent bacteria that forms the human skin microbiota. Specific phylotypes of C. acnes have been associated with the development of acne vulgaris, while other phylotypes have been linked to healthy skin. In this scenario, bacterial extracellular vesicles (EVs) play a role in the interkingdom communication role with the human host. The purpose of this study was to examine the impact of EVs generated by various phylotypes of C. acnes on inflammation and sebum production using different in vitro skin cell types. The main findings of this study reveal that the proteomic profile of the cargo embodied in the EVs reflects distinct characteristics of the different C. acnes phylotypes in terms of life cycle, survival, and virulence. The in vitro skin cell types showed an extended pro-inflammatory modulation of SLST A1 EVs consistently triggering the activation of the inflammation-related factors IL-8, IL-6, TNFα and GM-CSF, in comparison to SLST H1 and SLST H2. Additionally, an acne-prone skin model utilizing PCi-SEB and arachidonic acid as a sebum inducer, was employed to investigate the impact of C. acnes EVs on sebum regulation. Our findings indicated that all three types of EVs significantly inhibited sebum production after a 24-h treatment period, with SLST H1 EVs exhibiting the most pronounced inhibitory effect when compared to the positive control. The results of this study highlight the protective nature of C. acnes SLST H1 EVs and their potential use as a natural treatment option for alleviating symptoms associated with inflammation and oily skin.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Pro-inflammatory activity of Cutibacterium acnes phylotype IA1 and extracellular vesicles: An in vitro study.Exp Dermatol. 2024 Aug;33(8):e15150. doi: 10.1111/exd.15150. Exp Dermatol. 2024. PMID: 39113601 Free PMC article.
-
Potential functionality of Cutibacterium acnes extracellular vesicles in atopic dermatitis and acne vulgaris: A comparative proteomic analysis.Proteomics Clin Appl. 2024 Sep;18(5):e2300106. doi: 10.1002/prca.202300106. Epub 2024 Apr 19. Proteomics Clin Appl. 2024. PMID: 38639920
-
Different Cutibacterium acnes Phylotypes Release Distinct Extracellular Vesicles.Int J Mol Sci. 2022 May 21;23(10):5797. doi: 10.3390/ijms23105797. Int J Mol Sci. 2022. PMID: 35628607 Free PMC article.
-
The increasing importance of the gut microbiome in acne vulgaris.Folia Microbiol (Praha). 2022 Dec;67(6):825-835. doi: 10.1007/s12223-022-00982-5. Epub 2022 Jun 16. Folia Microbiol (Praha). 2022. PMID: 35711021 Review.
-
Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates.J Eur Acad Dermatol Venereol. 2018 Jun;32 Suppl 2:5-14. doi: 10.1111/jdv.15043. J Eur Acad Dermatol Venereol. 2018. PMID: 29894579 Review.
Cited by
-
The emerging role of bacterial extracellular vesicles in human cancers.J Extracell Vesicles. 2024 Oct;13(10):e12521. doi: 10.1002/jev2.12521. J Extracell Vesicles. 2024. PMID: 39377479 Free PMC article. Review.
-
Taurine and Polyphenol Complex Repaired Epidermal Keratinocyte Wounds by Regulating IL8 and TIMP2 Expression.Curr Issues Mol Biol. 2024 Aug 8;46(8):8685-8698. doi: 10.3390/cimb46080512. Curr Issues Mol Biol. 2024. PMID: 39194729 Free PMC article.
-
Antibacterial and hemocompatibility potentials of nano-gold-cored alginate preparation against anaerobic bacteria from acne vulgaris.Sci Rep. 2024 Mar 24;14(1):6984. doi: 10.1038/s41598-024-57643-5. Sci Rep. 2024. PMID: 38523189 Free PMC article.
-
Antibacterial activity and impact on keratinocyte cell growth of Cutibacterium acnes bacteriophages in a Cutibacterium acnes IA1- colonized keratinocyte model.Curr Res Microb Sci. 2025 Jan 30;8:100356. doi: 10.1016/j.crmicr.2025.100356. eCollection 2025. Curr Res Microb Sci. 2025. PMID: 39995444 Free PMC article.
-
Peiminine Exerts Its Anti-Acne Effects by Regulating the NF-κB Pathway.Antioxidants (Basel). 2024 Jan 22;13(1):131. doi: 10.3390/antiox13010131. Antioxidants (Basel). 2024. PMID: 38275656 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

