Efficient targeting of HIF-1α mediated by YC-1 and PX-12 encapsulated niosomes: potential application in colon cancer therapy
- PMID: 37749603
- PMCID: PMC10521571
- DOI: 10.1186/s13036-023-00375-3
Efficient targeting of HIF-1α mediated by YC-1 and PX-12 encapsulated niosomes: potential application in colon cancer therapy
Abstract
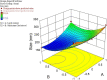
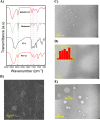
A number of molecular biofactors have been documented in pathogenesis and poor prognosis of colorectal cancer (CRC). Among them, the Hypoxia-Inducible Factor (HIF-1a) is frequently reported to become over-expressed, and its targeting could restrict and control a variety of essential hallmarks of CRC. Niosomes are innovative drug delivery vehicles with the encapsulating capacity for co-loading both hydrophilic and hydrophobic drugs at the same time. Also, they can enhance the local accumulation while minimizing the dose and side effects of drugs. YC-1 and PX-12 are two inhibitors of HIF-1a. The purpose of this work was to synthesize dual-loaded YC-1 and PX-12 niosomes to efficiently target HIF-1α in CRC, HT-29 cells. The niosomes were prepared by the thin-film hydration method, then the niosomal formulation of YC-1 and PX-12 (NIO/PX-YC) was developed and optimized by the central composition method (CCD) using the Box-Behnken design in terms of size, polydispersity index (PDI), entrapment efficiency (EE). Also, they are characterized by DLS, FESEM, and TEM microscopy, as well as FTIR spectroscopy. Additionally, entrapment efficiency, in vitro drug release kinetics, and stability were assessed. Cytotoxicity, apoptosis, and cell cycle studies were performed after the treatment of HT-29 cells with NIO/PX-YC. The expression of HIF-1αat both mRNA and protein levels were studied after NIO/PX-YC treatment. The prepared NIO/PX-YC showed a mean particle size of 185 nm with a zeta potential of about-7.10 mv and a spherical morphology. Also, PX-12 and YC-1 represented the entrapment efficiency of about %78 and %91, respectively, with a sustainable and controllable release. The greater effect of NIO/PX-YC than the free state of PX-YC on the cell survival rate, cell apoptosis, and HIF-1α gene/protein expression were detected (p < 0.05). In conclusion, dual loading of niosomes with YC-1 and PX-12 enhanced the effect of drugs on HIF-1α inhibition, thus boosting their anticancer effects.
Keywords: Colon cancer; Drug delivery; HIF-1α; Niosome; PX-12; Targeting; YC-1.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Targeting Colorectal Cancer Cells with Niosomes Systems Loaded with Two Anticancer Drugs Models; Comparative In Vitro and Anticancer Studies.Pharmaceuticals (Basel). 2022 Jun 30;15(7):816. doi: 10.3390/ph15070816. Pharmaceuticals (Basel). 2022. PMID: 35890115 Free PMC article.
-
Synergistic effect of curcumin-Cu and curcumin-Ag nanoparticle loaded niosome: Enhanced antibacterial and anti-biofilm activities.Bioorg Chem. 2021 Oct;115:105116. doi: 10.1016/j.bioorg.2021.105116. Epub 2021 Jun 24. Bioorg Chem. 2021. PMID: 34333420
-
Dermal targeting of Centella asiatica extract using hyaluronic acid surface modified niosomes.J Liposome Res. 2020 Jun;30(2):197-207. doi: 10.1080/08982104.2019.1614952. Epub 2019 May 27. J Liposome Res. 2020. PMID: 31060402
-
Advances in antitumor research of HIF-1α inhibitor YC-1 and its derivatives.Bioorg Chem. 2023 Apr;133:106400. doi: 10.1016/j.bioorg.2023.106400. Epub 2023 Jan 30. Bioorg Chem. 2023. PMID: 36739684 Review.
-
Niosome Preparation Techniques and Structure-An Illustrated Review.Pharmaceutics. 2025 Jan 6;17(1):67. doi: 10.3390/pharmaceutics17010067. Pharmaceutics. 2025. PMID: 39861715 Free PMC article. Review.
Cited by
-
Niosomes as Vesicular Nanocarriers in Cosmetics: Characterisation, Development and Efficacy.Pharmaceutics. 2025 Feb 21;17(3):287. doi: 10.3390/pharmaceutics17030287. Pharmaceutics. 2025. PMID: 40142950 Free PMC article. Review.
-
Curcumin and Silibinin loaded pegylated nanoniosome for cancer therapy: bioinformatics and in vitro study.Mol Biol Rep. 2025 Jul 19;52(1):737. doi: 10.1007/s11033-025-10835-2. Mol Biol Rep. 2025. PMID: 40682596
-
The role of hypoxic microenvironment in autoimmune diseases.Front Immunol. 2024 Nov 7;15:1435306. doi: 10.3389/fimmu.2024.1435306. eCollection 2024. Front Immunol. 2024. PMID: 39575238 Free PMC article. Review.
-
HIF-1α: A Key Factor Mediating Tumor Cells from Digestive System to Evade NK Cell Killing via Activating Metalloproteinases to Hydrolyze MICA/B.Biomolecules. 2025 Jun 19;15(6):899. doi: 10.3390/biom15060899. Biomolecules. 2025. PMID: 40563539 Free PMC article. Review.
-
Hypoxia inducible factor 1-alpha in the pathogenesis of abdominal aortic aneurysms in vivo: A narrative review.JVS Vasc Sci. 2023 Dec 26;5:100189. doi: 10.1016/j.jvssci.2023.100189. eCollection 2024. JVS Vasc Sci. 2023. PMID: 38379781 Free PMC article. Review.
References
-
- Serini S, Cassano R, Corsetto PA, Rizzo AM, Calviello G, Trombino S. Omega-3 PUFA loaded in resveratrol-based solid lipid nanoparticles: physicochemical properties and antineoplastic activities in human colorectal cancer cells in vitro. Int J Mol Sci. 2018;19(2):586. doi: 10.3390/ijms19020586. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

