Tyrosine-kinase inhibitor combined with iodine-125 seed brachytherapy for hepatocellular carcinoma refractory to transarterial chemoembolization: a propensity-matched study
- PMID: 37749616
- PMCID: PMC10518921
- DOI: 10.1186/s40644-023-00604-4
Tyrosine-kinase inhibitor combined with iodine-125 seed brachytherapy for hepatocellular carcinoma refractory to transarterial chemoembolization: a propensity-matched study
Abstract
Purpose: To investigate the efficacy and safety of tyrosine-kinase inhibitor (TKI) combined with iodine-125 seed brachytherapy (TKI-I) versus TKI alone for patients with hepatocellular carcinoma (HCC) refractory to transarterial chemoembolization (TACE).
Methods: Data of patients with TACE-refractory HCC who received TKI (sorafenib or lenvatinib) or TKI-I from September 2018 to December 2020 were retrospectively analyzed. A propensity score matching (PSM) was performed to diminish potential bias. The primary endpoints were overall survival (OS) and time to progression (TTP). Tumor responses and treatment-related adverse events (TRAEs) were also compared between the two groups.
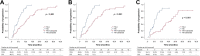
Results: A total of 132 patients were included in this study. Under PSM, 48 paired patients were selected for comparison. The median OS was 23.2 (95% CI 20.9-25.1) months in the TKI-I group versus 13.9 (95% CI 11.1-16.7) months in the TKI group (P < 0.001). The median TTP was 12.8 (95% CI 10.1-15.5) months in the TKI-I group versus 5.8 (95% CI 5.0-6.6) months in the TKI group (P < 0.001). Patients in the TKI-I group had higher objective response rate (68.8% vs. 33.3%, P = 0.001) and disease control rate (89.6% vs. 66.7%, P = 0.007) than those in the TKI group. The incidence and severity of TRAEs in the TKI-I group were comparable to those in the TKI group (any grade, 89.7% vs. 92.2%, P = 0.620; ≥grade 3, 33.8% vs. 32.8%, P = 0.902).
Conclusions: TKI-I was safe and significantly improved survival over TKI alone in HCC patients with TACE refractoriness.
Keywords: Brachytherapy; Combined modality therapy; Hepatocellular carcinoma; Therapeutic chemoembolization; Tyrosine kinase inhibitor.
© 2023. International Cancer Imaging Society (ICIS).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Lu J, Zhao M, Arai Y, Zhong BY, Zhu HD, Qi XL, et al. Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of International Society of Multidisciplinary Interventional Oncology (ISMIO) Hepatobiliary Surg Nutr. 2021;10:661–71. doi: 10.21037/hbsn-21-260. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82172043/National Natural Science Foundation of China
- 81873920/National Natural Science Foundation of China
- 202102010082/Guangzhou Municipal Science and Technology Project
- 202002030135/Guangzhou Municipal Science and Technology Project
- 202102020393/Guangzhou Municipal Science and Technology Project
- 0F04022/Clinical Featured Technology Program of Guangzhou
- 2023.198/Plan on Enhancing Scientific Research in GMU
- 2020KQNCX057/Young Innovative Talents Program of Guangdong Institutions of Higher Learning
- 320.6750.2022-09-2/Wu Jieping Medical Foundation
- 320.6750.2020-10-63/Wu Jieping Medical Foundation
- CXPJJH11900009-01/Chen Xiao-Ping Foundation for the Development of Science and Technology of Hubei Province
- CXPJJH1200008-03/Chen Xiao-Ping Foundation for the Development of Science and Technology of Hubei Province
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

