Lactam-fused tropolones: a new tunable, environmentally sensitive fluorophore class
- PMID: 37750360
- PMCID: PMC12309396
- DOI: 10.1039/d3ob01263h
Lactam-fused tropolones: a new tunable, environmentally sensitive fluorophore class
Abstract
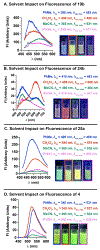
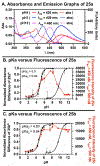
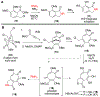
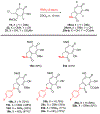
Fluorescent small-molecules capable of altering their profiles in response to environmental changes are exceptionally valuable tool compounds throughout the scientific community. The following manuscriipt describes a new class of fluorescent small molecules based on lactam-fused tropolones that are responsive to a dynamic range of environmental changes. These molecules can be easily obtained through a rapid annulation procedure between appropriately functionalized tropolones and primary amines, which is often complete within minutes at room temperature. Molecules generated through this approach have been identified with fluoresence emission across the visible light spectra, and can be tuned based on either the tropolone or amine component. They are also highly responsive to changes in solvent, pH, and certain divalent metal ions. Tropolone-fused lactams thus represent a new class of tunable fluorescent small molecules that could find value throughout the scientific community.
Conflict of interest statement
Conflict of Interest Statement
None
Figures








Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
"In a State of Flow": A Qualitative Examination of Autistic Adults' Phenomenological Experiences of Task Immersion.Autism Adulthood. 2024 Sep 16;6(3):362-373. doi: 10.1089/aut.2023.0032. eCollection 2024 Sep. Autism Adulthood. 2024. PMID: 39371355
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
Grants and funding
LinkOut - more resources
Full Text Sources

