Dravet syndrome: A systematic literature review of the illness burden
- PMID: 37750463
- PMCID: PMC10690674
- DOI: 10.1002/epi4.12832
Dravet syndrome: A systematic literature review of the illness burden
Abstract
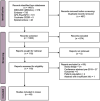
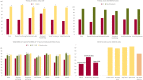
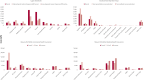
We performed a systematic literature review and narrative synthesis according to a pre-registered protocol (Prospero: CRD42022376561) to identify the evidence associated with the burden of illness in Dravet syndrome (DS), a developmental and epileptic encephalopathy characterized by drug-resistant epilepsy with neurocognitive and neurobehavioral impairment. We searched MEDLINE, Embase, and APA PsychInfo, Cochrane's database of systematic reviews, and Epistemonikos from inception to June 2022. Non-interventional studies reporting on epidemiology (incidence, prevalence, and mortality), patient and caregiver health-related quality of life (HRQoL), direct and indirect costs and healthcare resource utilization were eligible. Two reviewers independently carried out the screening. Pre-specified data were extracted and a narrative synthesis was conducted. Overall, 49 studies met the inclusion criteria. The incidence varied from 1:15 400-1:40 900, and the prevalence varied from 1.5 per 100 000 to 6.5 per 100 000. Mortality was reported in 3.7%-20.8% of DS patients, most commonly due to sudden unexpected death in epilepsy and status epilepticus. Patient HRQoL, assessed by caregivers, was lower than in non-DS epilepsy patients; mean scores (0 [worst] to 100/1 [best]) were 62.1 for the Kiddy KINDL/Kid-KINDL, 46.5-54.7 for the PedsQL and 0.42 for the EQ-5D-5L. Caregivers, especially mothers, were severely affected, with impacts on their time, energy, sleep, career, and finances, while siblings were also affected. Symptoms of depression were reported in 47%-70% of caregivers. Mean total direct costs were high across all studies, ranging from $11 048 to $77 914 per patient per year (PPPY), with inpatient admissions being a key cost driver across most studies. Mean costs related to lost productivity were only reported in three publications, ranging from approximately $19 000 to $20 000 PPPY ($17 596 for mothers vs $1564 for fathers). High seizure burden was associated with higher resource utilization, costs and poorer HRQoL. The burden of DS on patients, caregivers, the healthcare system, and society is profound, reflecting the severe nature of the syndrome. Future studies will be able to assess the impact that newly approved therapies have on reducing the burden of DS.
Keywords: caregiver burden; developmental and epileptic encephalopathy; direct costs; health-related quality of life; indirect costs.
© 2023 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
AS reports personal fees and grants from Angelini Pharma (Arvelle), Biocodex, Desitin Arzneimittel, Eisai, Jazz (GW) Pharmaceuticals, Marinus Pharma, Precisis, Takeda, UCB Pharma (Zogenix), and UNEEG medical. LL received grants, and is a consultant and/or speaker for Zogenix; LivaNova, UCB, Shire, Eisai, Novartis, Takeda/Ovid, Epihunter, Jazz Pharmaceuticals. JW receives an honorarium from Epillepsa for her work as an associate editor, she serves on the national (South African) advisory board for Sanofi. AB has received honoraria for presenting at educational events, advisory boards and consultancy work for Biocodex, Jazz Pharmaceuticals/GW Pharma, Encoded Therapeutics, Stoke Therapeutics and UCB/Zogenix. PS reports personal fees and grants from Angelini Pharma, Eisai, Jazz Pharmaceuticals Biomarin, UCB, Proveca, and Zogenix. FR reports personal fees from Angelini Pharma, Arvelle Therapeutics, Desitin Pharma, Eisai GmbH, GW Pharmaceuticals companies/Jazz Pharma, Roche Pharma and UCB, and grants from the Detlev-Wrobel-Fonds for Epilepsy Research, the German Research Foundation, the German Ministry of Education and Research, the LOEWE Programme of the State of Hesse, and the European Union. SSB reports personal fees from Eisai, Desitin Pharma, GW Pharmaceuticals companies, Ethypharm, UCB, and Zogenix. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures

References
-
- Strzelczyk A, Schubert‐Bast S. Therapeutic advances in Dravet syndrome: a targeted literature review. Exp Rev Neurotherapeut. 2020;20:1065–1079. - PubMed
-
- Zuberi SM, Wirrell E, Yozawitz E, Wilmshurst JM, Specchio N, Riney K, et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63:1349–1397. 10.1111/epi.17239 - DOI - PubMed
-
- Marini C, Mei D, Temudo T, Ferrari AR, Buti D, Dravet C, et al. Idiopathic epilepsies with seizures precipitated by fever and SCN1A abnormalities. Epilepsia. 2007;48:1678–1685. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

