Autoinflation for otitis media with effusion (OME) in children
- PMID: 37750500
- PMCID: PMC10521168
- DOI: 10.1002/14651858.CD015253.pub2
Autoinflation for otitis media with effusion (OME) in children
Abstract
Background: Otitis media with effusion (OME) is an accumulation of fluid in the middle ear cavity, common amongst young children. The fluid may cause hearing loss. When persistent, it may lead to behavioural problems and a delay in expressive language skills. Management of OME includes watchful waiting, medical, surgical and mechanical treatment. Autoinflation is a self-administered technique, which aims to ventilate the middle ear and encourage middle ear fluid clearance by providing a positive pressure of air in the nose and nasopharynx (using a nasal balloon or other handheld device). This positive pressure (sometimes combined with simultaneous swallow) encourages opening of the Eustachian tube and may help ventilate the middle ear.
Objectives: To assess the efficacy (benefits and harms) of autoinflation for the treatment of otitis media with effusion in children.
Search methods: The Cochrane ENT Information Specialist searched the Cochrane ENT Register; Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid Embase; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 20 January 2023.
Selection criteria: We included randomised controlled trials (RCTs) and quasi-randomised trials in children aged 6 months to 12 years with unilateral or bilateral OME. We included studies that compared autoinflation with either watchful waiting (no treatment), non-surgical treatment or ventilation tubes.
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were determined following a multi-stakeholder prioritisation exercise and were: 1) hearing, 2) OME-specific quality of life and 3) pain and distress. Secondary outcomes were: 1) persistence of OME, 2) other adverse effects (including eardrum perforation), 3) compliance or adherence to treatment, 4) receptive language skills, 5) speech development, 6) cognitive development, 7) psychosocial skills, 8) listening skills, 9) generic health-related quality of life, 10) parental stress, 11) vestibular function and 12) episodes of acute otitis media. We used GRADE to assess the certainty of evidence for each outcome. Although we included all measures of hearing assessment, the proportion of children who returned to normal hearing was our preferred method to assess hearing, due to challenges in interpreting the results of mean hearing thresholds.
Main results: We identified 11 completed studies that met our inclusion criteria (1036 participants). The majority of studies included children aged between 3 and 11 years. Most were carried out in Europe or North America, and they were conducted in both hospital and community settings. All compared autoinflation (using a variety of different methods and devices) to no treatment. Most studies required children to carry out autoinflation two to three times per day, for between 2 and 12 weeks. The outcomes were predominantly assessed just after the treatment phase had been completed. Here we report the effects at the longest follow-up for our main outcome measures. Return to normal hearing The evidence was very uncertain regarding the effect of autoinflation on the return to normal hearing. The longest duration of follow-up was 11 weeks. At this time point, the risk ratio was 2.67 in favour of autoinflation (95% confidence interval (CI) 1.73 to 4.12; 85% versus 32%; number needed to treat to benefit (NNTB) 2; 1 study, 94 participants), but the certainty of the evidence was very low. Disease-specific quality of life Autoinflation may result in a moderate improvement in quality of life (related to otitis media) after short-term follow-up. One study assessed quality of life using the Otitis Media Questionnaire-14 (OMQ-14) at three months of follow-up. Results were reported as the number of standard deviations above or below zero difference, with a range from -3 (better) to +3 (worse). The mean difference was -0.42 lower (better) for those who received autoinflation (95% CI -0.62 to -0.22; 1 study, 247 participants; low-certainty evidence; the authors report a change of 0.3 as clinically meaningful). Pain and distress caused by the procedure Autoinflation may result in an increased risk of ear pain, but the evidence was very uncertain. One study assessed this outcome, and identified a risk ratio of 3.50 for otalgia in those who received autoinflation, although the overall occurrence of pain was low (95% CI 0.74 to 16.59; 4.4% versus 1.3%; number needed to treat to harm (NNTH) 32; 1 study, 320 participants; very low-certainty evidence). Persistence of OME The evidence suggests that autoinflation may slightly reduce the persistence of OME at three months. Four studies were included, and the risk ratio for persistence of OME was 0.88 for those receiving autoinflation (95% CI 0.80 to 0.97; 4 studies, 483 participants; absolute reduction of 89 people per 1000 with persistent OME; NNTB 12; low-certainty evidence).
Authors' conclusions: All the evidence we identified was of low or very low certainty, meaning that we have little confidence in the estimated effects. However, the data suggest that autoinflation may have a beneficial effect on OME-specific quality of life and persistence of OME in the short term, but the effect is uncertain for return to normal hearing and adverse effects. The potential benefits should be weighed against the inconvenience of regularly carrying out autoinflation, and the possible risk of ear pain.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Katie Webster: none known.
Caroline A Mulvaney: none known.
Kevin Galbraith: none known.
Mridul Rana: none known.
Samuel MacKeith: treats patients with OME in his NHS and private practice and is Assistant Co‐ordinating Editor of Cochrane ENT but has not been involved in the editorial process for this review.
Tal Marom: treats patients with OME in his public sector and private practice.
Mat Daniel: treats patients with OME in his NHS practice. He has a financial interest in Aventamed, a company that produces a ventilation tube insertion device.
Roderick P Venekamp: is an Editor for Cochrane Acute Respiratory Infections and Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Professor Anne Schilder was joint Co‐ordinating Editor of Cochrane ENT until April 2020, but had no role in the editorial process for this review. She treats patients with OME in her NHS practice. Her evidENT team at the UCL Ear Institute is supported by the National Institute of Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC), with research projects being supported by the NIHR, Wellcome Trust, RNiD, ENT UK and industry. She is the National Specialty Lead for the NIHR Clinical Research Network ENT and Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Research Initiative. In her role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she advises CRO, biotech and pharma companies in the hearing field on clinical trial design and delivery.
Figures
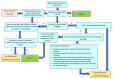
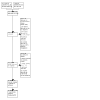

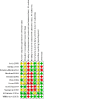



















Update of
- doi: 10.1002/14651858.CD015253
References
References to studies included in this review
Arick 2005 {published data only}
-
- Arick D, Silman S. Nonsurgical home treatment of middle ear effusion and associated hearing loss in children. Part I: clinical trial. Ear, Nose, & Throat Journal 2005;84(9):567-8, 570-4, 576 passim. [CENTRAL: CN-00552768] [EMBASE: 41864684] [PMID: ] - PubMed
Banigo 2016 {published data only}
-
- Banigo A, Hunt A, Rourke T, Whiteside O, Aldren C. Does the Earpopper(®) device improve hearing outcomes in children with persistent otitis media with effusion? A randomised single-blinded controlled trial. Clinical Otolaryngology 2016;41(1):59-65. [CENTRAL: CN-01078433] [EMBASE: 607683212] [PMID: ] - PubMed
Bidarian‐Moniri 2014 {published data only}
-
- Bidarian-Moniri A, Ramos MJ, Ejnell H. Autoinflation for treatment of persistent otitis media with effusion in children: a cross-over study with a 12-month follow-up. International Journal of Pediatric Otorhinolaryngology 2014;78(8):1298-305. [CENTRAL: CN-00994829] [EMBASE: 2014464001] [PMID: ] - PubMed
Blanshard 1993 {published data only}
-
- Blanshard J, Maw A, Bawden R. The treatment of otitis media with effusion in children by autoinflation. In: 2nd European Congress of Oto-Rhino-Laryngology and Cervico-facial Surgery. 1992:31-7. [CENTRAL: CN-01165327]
-
- Blanshard JD, Maw AR, Bawden R. Conservative treatment of otitis-media with effusion by autoinflation of the middle-ear. Clinical Otolaryngology 1993;18(3):188-92. [CENTRAL: CN-02472342] - PubMed
Brooker 1992 {published data only}
-
- Brooker DS, McNeice A. Autoinflation in the treatment of glue ear in children. Clinical Otolaryngology and Allied Sciences 1992;17(4):289-90. [CENTRAL: CN-00262418] [PMID: ] - PubMed
Chan 1989 {published data only}
-
- Chan KH, Bluestone CD. Lack of efficacy of middle-ear inflation: treatment of otitis media with effusion in children. Otolaryngology--Head and Neck Surgery 1989;100(4):317-23. [CENTRAL: CN-00060254] [PMID: ] - PubMed
Ercan 2005 {published data only}
-
- Ercan I, Cakir BC, Kayaoglu S, Turgut S. Long term effect of autoinflation in the treatment of otitis media with effusion. Elektronik Kulak Buran Bogaz ve Bas Boyun Cerrahisi Dergisi [Electronic Journal of Otolalryngology - Head and Neck Surgery] 2005;4(4):166-70. [CENTRAL: CN-00849154]
Scadding 2014 {published data only}
-
- Scadding G, Rajput K, Parikh A, Hilss S, Jansz J A, Darby YC, et al. Double blind, placebo-controlled study of Flixonase with and without Otovent in the treatment of otitis media with effusion. In: 8th International Congress of Paediatric Otorhinolaryngology (ESPO); 2002 Sep 11-14; Oxford, UK. 2002:92-3. [ABSTRACT NUMBER: 21.04] [CENTRAL: CN-00431597]
-
- Scadding GK, Darby YC, Jansz AJ, Richards D, Tate H, Hills S, et al. Double-blind, placebo controlled randomised trial of medical therapy in otitis media with effusion. Advances in Life Sciences and Health 2014;1(2):1-11. [CENTRAL: CN-01043521]
Stangerup 1992 {published data only}
-
- Stangerup S, Tos M. Autoinflation in the treatment of Eustachian tube dysfunction, secretory otitis media and its sequelae. In: Mogi G (editor), editors(s). Recent Advances in Otitis Media. Amsterdam/New York: Kugler Publications, 1994:759-63. [CENTRAL: CN-01214464]
-
- Stangerup SE, Sederberg-Olsen J, Balle V. Autoinflation as a treatment of secretory otitis media. A randomized controlled study. Archives of Otolaryngology--Head & Neck Surgery 1992;118(2):149-52. [CENTRAL: CN-00082084] [PMID: ] - PubMed
Williamson 2015a {published data only}ISRCTN55208702
-
- ISRCTN55208702. An open randomised study of autoinflation in 4-11 year old school children with otitis media with effusion (OME) in primary care [Study of autoinflation in 4-11 year old school children with glue ear]. https://www.isrctn.com/ISRCTN55208702 (first received 16 June 2011). [CENTRAL: CN-01012774]
-
- Williamson I, Vennik J, Harnden A, Voysey M, Perera R, Breen M, et al. An open randomised study of autoinflation in 4- to 11-year-old school children with otitis media with effusion in primary care. Health Technology Assessment (Winchester, England) 2015;19(72):1-150. [CENTRAL: CN-01098789] [EMBASE: 606141685] [PMID: ] - PMC - PubMed
-
- Williamson I, Vennik J, Harnden A, Voysey M, Perera R, Kelly S, et al. Effect of nasal balloon autoinflation in children with otitis media with effusion in primary care: an open randomized controlled trial. CMAJ: Canadian Medical Association Journal 2015;187(13):961-9. [CENTRAL: CN-01078404] [PMID: ] - PMC - PubMed
Williamson 2015b {published data only}
References to studies excluded from this review
Ardehali 2008 {published data only}
-
- Ardehali MM, Seraj JM, Asiabar MK, Adibi H. The possible role of gastroesophageal reflux disease in children suffering from chronic otitis media with effusion. Acta Medica Iranica 2008;46(1):33-7. [CENTRAL: CN-00708224] [EMBASE: 351792703]
Bidarian‐Moniri 2016 {published data only}
ChiCTR2000035008 {published data only}
-
- ChiCTR2000035008. Multicenter randomized controlled study of APONSTEM eustachian tube balloon as a treatment for children with recurrent chronic secretory otitis media. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000035008 (first received 2020). [CENTRAL: CN-02184651]
Choung 2008 {published data only}
De Nobili 2008 {published data only}
-
- De Nobili E, Bellomo A, De Nobili E, Bellomo A. Comparative evaluation of efficacy of crenotherapeutic Politzer with sulphurous water versus crenotherapeutic Politzer and autoinsufflation (Otovent) in patients with tubaric dysfunction and secretory otitis media [Studio comparativo dell’efficacia del Politzer crenoterapico con acqua sulfurea versus Politzer crenoterapico e autoinsufflazione domiciliare (metodo Otovent®) in pazienti affetti da disfunzione tubarica e otite media secretiva]. Medicina Clinica e Termale 2008;20(64):30-4. [CENTRAL: CN-00675975]
El Hachem 2012 {published data only}
-
- El Hachem N, Halimi M. New technique for the passive opening of the Eustachian tube. Tympanometry assessment of the effect of a new manipulation technique on the opening of the Eustachian tube in children less than six years with otitis media with or without effusion [Nouvelle technique passive d'ouverture de la trompe d'Eustache. Evaluation par tympanometrie de l'effet d'une nouvelle manipulation sur l'ouverture de la trompe d'Eustache chez les enfants de moins de six ans atteints d'une otite moyenne avec ou sans effusion]. Kinesitherapie 2012;132:25-33.
Endo 1997 {published data only}
-
- Endo LH, Antunes AB, Vidolin C, Bilecki MM, Magalhaes KVB. Secretory media otitis: clinical treatment vs placebo [Otite media secretora: tratamento clinco versus placebo]. Revista Brasileira de Otorrinolaringologia 1997;63(2):116-9. [CENTRAL: CN-00187118] [EMBASE: 27225833]
Ferrara 2005 {published data only}
-
- Ferrara S, Sammartano D, Ferrara P. Long-term management of recurrent otitis media with effusion in children. In: XVIII International Federation of Otorhinolaryngology Societies (IFOS) World Congress 2005 Jun 25-30; Rome (Italy). 2005. [CENTRAL: CN-00526409]
Gibson 1996 {published data only}
-
- Gibson PG, Stuart JE, Wlodarczyk J, Olson LG, Hensley MJ. Nasal inflammation and chronic ear disease in Australian Aboriginal children. Journal of Paediatrics and Child Health 1996;32(2):143-7. [CENTRAL: CN-00131589] [PMID: ] - PubMed
Head 1992 {published data only}
-
- Head M. Does nose blowing help hearing in glue ear? Nursing Times 1992;88(3):53. [CENTRAL: CN-00081595] [PMID: ] - PubMed
Heaf 1991 {published data only}
Iino 1989 {published data only}
-
- Iino Y, Ishitoya J, Ikeda M, Ito Y, Usami M, Kawashiro N, et al. Factors on delayed recovery of otitis media with effusion in children--clinical and bacteriological study. Nihon Jibiinkoka Gakkai Kaiho 1989;92(8):1183-91. [CENTRAL: CN-00063873] [PMID: ] - PubMed
Leunig 1995 {published data only}
-
- Leunig A, Mees K. Autoinflation of the middle ear with Otovent [Mittelohrbeluftung mit dem otovent-latexmembran-system]. Laryngo- Rhino- Otologie 1995;74(6):352-4. [CENTRAL: CN-00392275] [EMBASE: 25203663] - PubMed
Li 2020 {published data only}
-
- Li H, Zhou Y, Huang J, Zhang L, Lou Z. Treatment of secretory otitis media with balloon dilation and tympanostomy tube insertion: a randomized controlled trial. Basic & Clinical Pharmacology & Toxicology 2020;127(Suppl 1):292-3. [CENTRAL: CN-02230226] [EMBASE: 633830686]
Li 2021 {published data only}
-
- Li SN, Huang YY, Hou SL, Wu Y, Shen JL, Wang L, et al. Effect of autoflation on the prognosis of otitis media with effusion in children. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke za Zhi [Chinese Journal of Otorhinolaryngology Head and Neck Surgery] 2021;56(6):573-8. [PMID: ] - PubMed
Marchisio 1998 {published data only}
-
- Marchisio P, Principi N, Passali D, Salpietro DC, Boschi G, Chetri G, et al. Epidemiology and treatment of otitis media with effusion in children in the first year of primary school. Acta Oto-laryngologica 1998;118(4):557-62. [CENTRAL: CN-00154484] [EMBASE: 1998254893] [PMID: ] - PubMed
NCT03534219 {published data only}
-
- NCT03534219. Efficacy of the Earpopper device in children with recurrent otitis media [Efficacy of the Earpopper device in children with recurrent otitis media: a randomized controlled trial]. https://clinicaltrials.gov/show/nct03534219 (first received 11 May 2018). [CENTRAL: CN-01601566]
Paradise 1997 {published data only}
-
- Paradise J, Campbell T, Dollaghan C, Feldman H, Bernard B, Colborn K, et al. Receptive vocabulary, cognition, and parent-rated behavior at age 3 years in relation to otitis media in the first 3 years of life. In: Abstract Book of the Association of Health Service Research. Vol. 14. 1997:350-1. [CENTRAL: CN-00452820]
Parlea 2012 {published data only}
Rohail 2006 {published data only}
-
- Rohail A, Gill ZI, Butt MR. A comparison of medical treatment versus surgical treatment for the management of otitis media with effusion. Annals of King Edward Medical College 2006;12(1):64-7. [CENTRAL: CN-00597368]
Shubich 1996 {published data only}
-
- Shubich I. Otitis media with effusion and allergy control in children: a prospective study. In: Sixth International Symposium on Otitis Media; 1996; Fort Lauderdale (FL). 1996:173-4. [CENTRAL: CN-00452904]
Silman 2005 {published data only}
-
- Silman S, Arick DS, Emmer MB. Nonsurgical home treatment of middle ear effusion and associated hearing loss in children. Part II: validation study. Ear Nose & Throat Journal 2005;84(10):646-50. [CENTRAL: CN-00597170] [PMID: ] - PubMed
Starcevic 2011 {published data only}
-
- Velepic M, Starcevic R, Bonifacic M, Ticac R, Kujundzic M, Udovic DS, et al. The clinical status of the eardrum: an inclusion criterion for the treatment of chronic secretory otitis media in children. International Journal of Pediatric Otorhinolaryngology 2011;75(5):686-90. [CENTRAL: CN-00784332] [EMBASE: 51315541] [PMID: ] - PubMed
Stenstrom 2005 {published data only}
-
- Stenstrom R, Pless IB, Bernard P. Hearing thresholds and tympanic membrane sequelae in children managed medically or surgically for otitis media with effusion. Archives of Pediatrics & Adolescent Medicine 2005;159(12):1151-6. [CENTRAL: CN-00532329] [PMID: ] - PubMed
Tham 2018 {published data only}
References to studies awaiting assessment
Tawfik 2002 {published data only}
-
- Tawfik S, Belal A, Sorour W. A comparative study of the different treatment modalities of otitis media with effusion in children. In: 8th International Congress of Paediatric Otorhinolaryngology (ESPO). Oxford, UK, 11-14 September, 2002. 2002:151. [ABSTRACT NUMBER: P2.26] [CENTRAL: CN-00508402]
References to ongoing studies
INFLATE (ACTRN12617001652369) {published data only}
-
- ACTRN12617001652369. Autoinflation for Aboriginal and Torres Strait Islander children with bilateral OME (middle ear infection) [A multi-centre randomised controlled trial to compare the efficacy of nasal balloon autoinflation versus no nasal balloon autoinflation for bilateral otitis media with effusion in Aboriginal and Torres Strait Islander children]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001652369 (first received 2017). [CENTRAL: CN-01896354]
-
- Walsh R, Reath J, Gunasekera H, Leach A, Kong K, Askew D, et al. INFLATE: a protocol for a randomised controlled trial comparing nasal balloon autoinflation to no nasal balloon autoinflation for otitis media with effusion in Aboriginal and Torres Strait Islander children. Trials 2022;23(1):309. - PMC - PubMed
NCT00393159 {published data only}
-
- NCT00393159. The influence of The Ear Popper on serous otitis media and on the accompanying conductive hearing loss in children. https://clinicaltrials.gov/show/NCT00393159 (first received 26 October 2006). Accessed 15/01/2023. [CENTRAL: CN-01038104]
NCT02038400 {published data only}
-
- NCT02038400. Evaluation of the efficiency of KINETUBE® in the recurrent chronic tubal ear otitis care (otitis media with effusion, atelectatic otitis or retraction) in children with an age range of 7-15 years. https://clinicaltrials.gov/show/NCT02038400 (first received 16 January 2014). Accessed 15/01/2023. [CENTRAL: CN-00921546]
NCT02546518 {published data only}
-
- NCT02546518. A comparison of surgical and a new non-surgical treatment methods for secretory otitis media in children- hearing and socioeconomic aspects. https://clinicaltrials.gov/show/nct02546518 (first received 11 September 2015). Accessed 15/01/2023. [CENTRAL: CN-01102072]
NCT05324696 {published data only}
-
- NCT05324696. Autoinflation: alternative in the treatment of otitis media with effusion (OME). https://clinicaltrials.gov/show/NCT05324696 (first received 12 April 2022). Accessed 15/01/2023.
Additional references
Abidin 1995
-
- Abidin RR. Parenting Stress Index: Professional Manual. Third edition. Odessa, FL: Psychological Assessment Resources, 1995.
Achenbach 2011
-
- Achenbach TM. Child Behavior Checklist. In: Kreutzer JS, DeLuca J, Caplan B, editors(s). Encyclopedia of Clinical Neuropsychology. New York, NY: Springer, 2011. [DOI: 10.1007/978-0-387-79948-3_1529] - DOI
Bayley 2006
-
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: Harcourt Assessment, Inc, 2006.
Berkman 2013
-
- Berkman ND, Wallace IF, Steiner MJ, Harrison M, Greenblatt AM, Lohr KN, et al. Otitis media with effusion: comparative effectiveness of treatments [Internet]. Report No.: 13-EHC091-EF edition. Vol. 1. Rockville (MD): Agency for Healthcare Research and Quality (US), 2013. - PubMed
Bruce 2015
Cochrane ENT 2020
-
- Cochrane ENT. Otitis media with effusion: a project to prioritise Cochrane systematic reviews. https://ent.cochrane.org/otitis-media-effusion-ome-glue-ear 2020 (accessed 3 November 2021).
Dewey 2000
-
- Dewey C, Midgeley E, Maw R. The relationship between otitis media with effusion and contact with other children in a british cohort studied from 8 months to 3 1/2 years. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. International Journal of Pediatric Otorhinolaryngology 2000;55(1):33-45. - PubMed
Dunn 2007
-
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test, Fourth Edition (PPVT™-4). Pearson Education, 2007.
Egger 1997
Ejnell 2015a
-
- Ejnell H. A comparison of surgical and a new non-surgical treatment methods for secretory otitis media in children- hearing and socioeconomic aspects. https://clinicaltrials.gov/show/nct02546518 (first received 11 September 2015).
Ejnell 2015b
-
- Ejnell H. The effect of early non-surgical treatment of children with middle ear effusion on the hearing level and the health economics. https://clinicaltrials.gov/show/nct02549612 (first received 15 September 2015).
Fekkes 2000
Goodman 1997
Gresham 1990
-
- Gresham FM, Elliott SN. Social Skills Rating System. Circle Pines, MN: American Guidance Service, 1990.
Griffiths 1996
-
- Griffiths R. The Griffiths Mental Development Scales from Birth to Two Years, manual, the 1996 revision. Henley: Association for Research in Infant and Child Development, Test Agency, 1996.
Haggard 2003
-
- Haggard MP, Smith SC, Nicholls EE. Quality of life and child behaviour. In: Rosenfeld RM, Bluestone CD, editors(s). Evidence-based Otitis Media. 2nd edition. Hamilton, Ontario: BC Decker Inc, 2003:401-29. [https://researchonline.lshtm.ac.uk/id/eprint/15108]
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hedrick 1984
-
- Hedrick DL, Prather EM, Tobin AR. Sequenced Inventory of Communication Development. Seattle, WA: University of Washington Press, 1984.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. - PMC - PubMed
Jellinek 1988
Kreiner‐Møller 2012
Landgraf 1994
-
- Landgraf JM. The Infant/Toddler Child Health Questionnaire: conceptual framework, logic content, and preliminary psychometric results. Boston: Health Act, 1994.
Landgraf 1996
-
- Landgraf JL, Abetz L, Ware JE. The CHQ User’s Manual. Boston: The Health Institute, New England Medical Center, 1996.
Lefebvre 2020
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Liu 2020
MacKeith 2022a
MacKeith 2022b
Marseglia 2008
Marshall 2018
Marx 1995
-
- Marx J, Osguthorpe JD, Parsons G. Day care and the incidence of otitis media in young children. Otolaryngology Head and Neck Surgery 1995;112(6):695-9. - PubMed
McCarthy 1972
-
- McCarthy D. Manual for the McCarthy Scales of Children's Abilities. New York: Psychological Corp, 1972.
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 Sep 13-17; Cape Town, South Africa. 2017.
Mulvaney 2022a
Mulvaney 2022b
NICE 2008
-
- NICE. Otitis media with effusion in under 12s: Otitis media with effusion in under 12s: surgery (CG60). Available at: https://www.nice.org.uk/guidance/cg60 2008 (accessed 3 November 2021).
NICE 2016
-
- National Institute for Health and Care Excellence. Otovent nasal balloon for otitis media with effusion. https://www.nice.org.uk/advice/mib59/resources/otovent-nasal-balloon-for... 2016 (accessed 3 November 2021).
NICE 2023
-
- National Institute for Health and Care Excellence. Otitis media with effusion in under 12s. NICE guideline [NG233]; August 2023. https://www.nice.org.uk/guidance/ng233. - PubMed
Noel‐Storr 2018
-
- Noel-Storr AH. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 Jun 18-20; Oxford, UK. 2018.
Perera 2013
Rabin 2001
RACGP 2016
-
- The Royal Australian College of General Practitioners. Autoinflation: glue ear. https://www.racgp.org.au/getattachment/14295770-1083-475d-b7ae-8d43d7ecf... 2016 (accessed 3 November 2021).
RevMan 2014 [Computer program]
-
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynell 1985
-
- Reynell JH. Reynell Development Language Scales Manual. 2nd edition. Windsor, UK: NFER-Nelson, 1985.
Rosenfeld 1997
Rosenfeld 2000
Rosenfeld 2003
-
- Rosenfeld RM, Kay D. Natural history of untreated otitis media. Laryngoscope 2003;113:1645-57. - PubMed
Rosenfeld 2016
Rovers 2006
-
- Rovers MM, Kok IM, Schilder AG. Risk factors for otitis media: an international perspective. International Journal of Pediatric Otorhinolaryngology 2006;70(7):1251-6. - PubMed
Schlichting 2007
-
- Schlichting JEPT, Lutje Spelberg HC. Lexilijst Begrip: An instrument to investigate language comprehension in children aged 15-25 months in the context of early identification. Amsterdam: Pearson Assessment & Information BV, 2007.
Schlichting 2010
-
- Schlichting JEPT, Lutje Spelberg HC. Schlichting Test for Language Comprehension; Instruction manual. Wooden: Bohn Stafleu van Loghum, 2010.
Schmalbach 2021
-
- Schmalbach CE, Brereton J, Bowman C, Denneny JC. American Academy of Otolaryngology–Head and Neck Surgery/Foundation Reg-ent Registry: Purpose, Properties, and Priorities. Otolaryngology – Head and Neck Surgery 2021;164(5):964-71. - PubMed
Thomas 2017
-
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al, Living Systematic Review Network. Living systematic reviews 2: combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. - PubMed
TNO 1997
-
- TNO—Prevention and Health/LUMC. TAIQOL—Questionnaire for parents of children aged 1—5 years. Leiden, The Netherlands: Leiden University Medical Center, 1997.
Verrips 1998
-
- Verrips GH, Vogels AGC, Verloove-Vanhorick SP, Fekkes M, Koopman HM, Kamphuis RP, et al. Health-related quality of life measure for children - the TACQOL. Journal of Applied Therapeutics 1998;1(4):357-60.
Wallace 2017
Wan 2014
Williamson 2011
Williamson 2015
-
- Williamson I, Vennik J, Harnden A, Voysey M, Perera R, Kelly S, et al. Effect of nasal balloon autoinflation in children with otitis media with effusion in primary care: an open randomized controlled trial. Canadian Medical Association Journal 2015;187(13):961-9. [DOI: 10.1503/ cmaj.141608] - PMC - PubMed
Zernotti 2017
Zimmerman 1992
-
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale-3. San Antonio, TX: The Psychological Corporation, 1992.
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

