Nomogram for Predicting Risk of Cancer Therapy-Related Cardiac Dysfunction in Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer
- PMID: 37750581
- PMCID: PMC10727240
- DOI: 10.1161/JAHA.123.029465
Nomogram for Predicting Risk of Cancer Therapy-Related Cardiac Dysfunction in Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer
Abstract
Background: Cancer therapy-related cardiac dysfunction (CTRCD) is an important treatment-limiting toxicity for patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer that adversely affects cancer and cardiovascular outcomes. Easy-to-use tools that incorporate readily accessible clinical variables for individual estimation of CTRCD risk are needed.
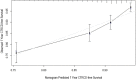
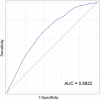
Methods and results: From 2004 to 2013, 1440 patients with stage I to III HER2-positive breast cancer treated with trastuzumab-based therapy were identified. A multivariable Cox proportional hazards model was constructed to identify risk factors for CTRCD and included the 1377 patients in whom data were complete. Nine clinical variables, including age, race, body mass index, left ventricular ejection fraction, systolic blood pressure, coronary artery disease, diabetes, arrhythmia, and anthracycline exposure were built into a nomogram estimating risk of CTRCD at 1 year. The nomogram was validated for calibration and discrimination using bootstrap resampling. A total of 177 CTRCD events occurred within 1 year of HER2-targeted treatment. The nomogram for prediction of 1-year CTRCD probability demonstrated good discrimination, with a concordance index of 0.687. The predicted and observed probabilities of CTRCD were similar, demonstrating good model calibration.
Conclusions: A nomogram composed of 9 readily accessible clinical variables provides an individualized 1-year risk estimate of CTRCD among women with HER2-positive breast cancer receiving HER2-targeted therapy. This nomogram represents a simple-to-use tool for clinicians and patients that can inform clinical decision-making on breast cancer treatment options, optimal frequency of cardiac surveillance, and role of cardioprotective strategies.
Keywords: breast cancer; cardiotoxicity; cardio‐oncology.
Figures


References
-
- Yu AF, Flynn JR, Moskowitz CS, Scott JM, Oeffinger KC, Dang CT, Liu JE, Jones LW, Steingart RM. Long‐term cardiopulmonary consequences of treatment‐induced cardiotoxicity in survivors of ERBB2‐positive breast cancer. JAMA Cardiol. 2020;5:309–317. doi: 10.1001/jamacardio.2019.5586 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

