Outpatient Treatment with AZD7442 (Tixagevimab/Cilgavimab) Prevented COVID-19 Hospitalizations over 6 Months and Reduced Symptom Progression in the TACKLE Randomized Trial
- PMID: 37751015
- PMCID: PMC10581960
- DOI: 10.1007/s40121-023-00861-7
Outpatient Treatment with AZD7442 (Tixagevimab/Cilgavimab) Prevented COVID-19 Hospitalizations over 6 Months and Reduced Symptom Progression in the TACKLE Randomized Trial
Abstract
Introduction: We assessed effects of AZD7442 (tixagevimab/cilgavimab) on deaths from any cause or hospitalizations due to coronavirus disease 2019 (COVID-19) and symptom severity and longer-term safety in the TACKLE adult outpatient treatment study.
Methods: Participants received 600 mg AZD7442 (n = 452) or placebo (n = 451) ≤ 7 days of COVID-19 symptom onset.
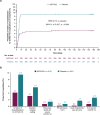
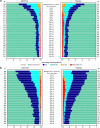
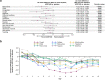
Results: Death from any cause or hospitalization for COVID-19 complications or sequelae through day 169 (key secondary endpoint) occurred in 20/399 (5.0%) participants receiving AZD7442 versus 40/407 (9.8%) receiving placebo [relative risk reduction (RRR) 49.1%; 95% confidence interval (CI) 14.5, 69.7; p = 0.009] or 50.7% (95% CI 17.5, 70.5; p = 0.006) after excluding participants unblinded before day 169 for consideration of vaccination). AZD7442 reduced progression of COVID-19 symptoms versus placebo through to day 29 (RRR 12.5%; 95% CI 0.5, 23.0) and improved most symptoms within 1-2 weeks. Over median safety follow-up of 170 days, adverse events occurred in 174 (38.5%) and 196 (43.5%) participants receiving AZD7442 or placebo, respectively. Cardiac serious adverse events occurred in two (0.4%) and three (0.7%) participants receiving AZD7442 or placebo, respectively.
Conclusions: AZD7442 was well tolerated and reduced hospitalization and mortality through 6 months, and symptom burden through 29 days, in outpatients with mild-to-moderate COVID-19.
Clinical trial registration: Clinicaltrials.gov, NCT04723394. ( https://beta.
Clinicaltrials: gov/study/NCT04723394 ).
Keywords: AZD7442; COVID-19; Cilgavimab; Hospitalization; Monoclonal antibodies; SARS-CoV-2; Symptoms; Tixagevimab.
Plain language summary
Antibodies are proteins produced by the body’s immune system to specifically combat foreign substances, such as viruses. Tixagevimab and cilgavimab are a pair of antibodies that bind to a specific part of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). When they bind to the virus, they reduce its ability to cause disease. These antibodies were tested in a clinical trial to see if they could prevent people with COVID-19 from being hospitalized or dying. Around 900 adults took part in this clinical trial. These people all had COVID-19 but were not sick enough to be in hospital. Half of this group were treated with a dose of tixagevimab and cilgavimab, given as two injections. The other half received a placebo (injections that look exactly like the tixagevimab and cilgavimab injections but contain no medicine). The study found that, over 6 months, people with COVID-19 who received tixagevimab and cilgavimab were less likely to need to go to hospital than people who received the placebo. They were also less likely to die of COVID-19. Tixagevimab and cilgavimab also helped to improve COVID-19 symptoms. People who received the antibodies saw their symptoms improve faster than people who received the placebo. They were also less likely to have symptoms that got worse. Most people felt better within 1–2 weeks of getting treatment. No safety issues were found with tixagevimab and cilgavimab compared with placebo.
© 2023. The Author(s).
Conflict of interest statement
F.D. Richard Hobbs reports funding from AstraZeneca to cover meeting attendances and the operationalization of TACKLE in the UK as the UK principal investigator; has received funding from UK Research and Innovation and the National Institute for Health and Care Research (NIHR) for national Urgent Public Health COVID-19 trials, and as director of the NIHR Applied Research Collaboration, Oxford Thames Valley, and investigator on the Oxford Biomedical Research Centre (BRC) and NIHR MedTech. Hugh Montgomery is supported by the NIHR’s BRC at University College London Hospitals. He has received consultation fees from AstraZeneca and has consulted for Millfield Medical Electronics Ltd. on the development of a new Closed Circuit Continuous Positive Airway Pressure machine. Francisco Padilla has received personal fees and grants from Amgen, AstraZeneca, Boehringer Ingelheim, Ferrer, Kowa, Medix, Merck, Merck Sharp and Dohme, Novartis, Pfizer, Sanofi, Servier, and Silanes. Jesus Abraham Simón-Campos reports serving on advisory boards for Atea and Eli Lilly; and serves as a speaker for Pfizer, AstraZeneca, Roche, and Regeneron. Kenneth Kim has received research grants for the conduct of the TACKLE trial; reports funding from Regeneron, Eli Lilly, Merck, Pfizer, and Adagio; and serves as a speaker for Regeneron. Douglas Arbetter, Venkatesh Pilla Reddy, Seth Seegobin, Katie Streicher, Rolando M. Viani and Mark T. Esser are employees of, and hold or may hold stock in, AstraZeneca. Kelly W. Padilla was an employee of AstraZeneca at the time of this study and is now an employee of Immunovant, New York, NY, USA. Kelly W. Padilla holds stock in AstraZeneca, GSK (prior employee) and Trulab Inc (board member). Gavin C.K.W. Koh was an employee of AstraZeneca at the time of this study and is now an employee of Generate Biomedicines, Somerville, MA, USA. Gavin C.K.W. Koh holds stock in GSK. Eva Johnsson was an employee of AstraZeneca at the time of this study and is now an employee of Grunenthal, Stockholm, Sweden. Alison Templeton was an employee of AstraZeneca at the time of this study and is now an employee of Regeneron Pharmaceuticals, Inc, Uxbridge, UK.
Figures
References
-
- Our World in Data. Coronavirus (COVID-19) hospitalizations. 2022. https://ourworldindata.org/covid-hospitalizations. Accessed 8 Nov 2022.
-
- Office for National Statistics. Direct and indirect health impacts of COVID-19 in England: emerging Omicron impacts. 2022. https://www.gov.uk/government/publications/direct-and-indirect-health-im.... Accessed 8 Nov 2022.
-
- Centers for Disease Control and Prevention. COVID-NET: COVID-19-associated hospitalization surveillance network. 2022. https://gis.cdc.gov/grasp/covidnet/covid19_5.html. Accessed 8 Nov 2022.
-
- Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case–control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

