Week 240 Efficacy and Safety of Fostemsavir Plus Optimized Background Therapy in Heavily Treatment-Experienced Adults with HIV-1
- PMID: 37751019
- PMCID: PMC10581994
- DOI: 10.1007/s40121-023-00870-6
Week 240 Efficacy and Safety of Fostemsavir Plus Optimized Background Therapy in Heavily Treatment-Experienced Adults with HIV-1
Abstract
Introduction: Efficacy and safety of the attachment inhibitor fostemsavir + optimized background therapy (OBT) were evaluated through 48 and 96 weeks in the phase 3 BRIGHTE trial in heavily treatment-experienced (HTE) adults failing their current antiretroviral regimen. Here, we report 240-week efficacy and safety of fostemsavir + OBT in adults with multidrug-resistant human immunodeficiency virus (HIV)-1 in BRIGHTE.
Methods: Heavily treatment-experienced adults failing their current regimen entered the randomized cohort (RC; 1-2 fully active antiretrovirals available) or non-randomized cohort (NRC; no fully active antiretrovirals available) and received open-label fostemsavir + OBT (starting Day 8 in RC and Day 1 in NRC). Endpoints included proportion with virologic response (HIV-1 RNA < 40 copies/mL, Snapshot), immunologic efficacy, and safety.
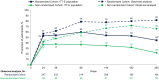
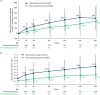
Results: At Week 240, 45% and 22% of the RC and NRC, respectively, had virologic response (Snapshot); 7% of the RC and 5% of the NRC had missing data due to coronavirus disease 2019 (COVID-19)-impacted visits. In the observed analysis, 82% of the RC and 66% of the NRC had virologic response. At Week 240, mean change from baseline in CD4+ T-cell count was 296 cells/mm3 (RC) and 240 cells/mm3 (NRC); mean CD4+/CD8+ ratio increased between Weeks 96 and 240 (RC 0.44 to 0.60; NRC 0.23 to 0.32). Between Weeks 96 and 240, four participants discontinued for adverse events, one additional participant experienced a drug-related serious adverse event, and six deaths occurred (median last available CD4+ T-cell count, 3 cells/mm3). COVID-19-related events occurred in 25 out of 371 participants; all resolved without incident.
Conclusion: Through ~5 years, fostemsavir + OBT demonstrated durable virologic and immunologic responses with no new safety concerns between Weeks 96 and 240, supporting this regimen as a key therapeutic option for HTE people with multidrug-resistant HIV-1.
Trial registration: ClinicalTrials.gov, NCT02362503.
Keywords: Advanced HIV disease; Attachment inhibitor; CD4+/CD8+ ratio; CD4+ T-cell count; Virologic response.
© 2023. The Author(s).
Conflict of interest statement
Judith A. Aberg has received grants from Emergent BioSolutions, Frontier Technologies, Gilead, GSK, Janssen, Merck, Pfizer, Regeneron, and ViiV Healthcare, which were paid to her institution, and participated in scientific advisory boards for GSK, Merck, and ViiV Healthcare. Bronagh Shepherd and Marcia Wang are employees of and may own stock in GSK. Jose V. Madruga has received grants for participation in scientific advisory boards from Gilead, GSK, Janssen, MSD, and ViiV Healthcare; sponsorship to attend international congresses from Gilead and Janssen; personal fees for lectures from Gilead, GSK, Janssen, MSD, Pfizer, and ViiV Healthcare; and has served as an investigator in clinical trials sponsored by Gilead, GSK, Janssen, MSD, Pfizer, Sanofi, and ViiV Healthcare. Fernando Mendo Urbina has received honoraria from Johnson and Johnson. Christine Katlama has received grants and/or personal fees from ViiV Healthcare, Gilead, and MSD. Shannon Schrader has received honoraria from Gilead and Janssen. Joseph J. Eron has received grants from Gilead, Janssen, and ViiV Healthcare, which were paid to his institution, and consultant fees from Gilead, Merck, and ViiV Healthcare. Princy N. Kumar has received grants from Eli Lilly, GSK, Merck, Gilead, Regeneron, American Gene Technologies, and BioHaven, which were paid to her institution; participated in data safety monitoring/advisory boards for Johnson & Johnson, ViiV Healthcare, Gilead, TheraTechnologies, and Merck; and holds stock/stock options in Merck, Johnson & Johnson, GSK, Gilead, Pfizer, and Moderna. Eduardo Sprinz has received grants for participation in scientific advisory boards from Abbott, Gilead, GSK, Janssen, MSD, Roche, and ViiV Healthcare. Margaret Gartland, Shiven Chabria, Andrew Clark, Amy Pierce, Max Lataillade, and Allan R. Tenorio are employees of ViiV Healthcare and may own stock in GSK.
Figures

References
-
- Rukobia [prescribing information]. Durham, NC: ViiV Healthcare; 2022.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

