Overall Survival Among Patients With De Novo Stage IV Metastatic and Distant Metastatic Recurrent Non-Small Cell Lung Cancer
- PMID: 37751203
- PMCID: PMC10523163
- DOI: 10.1001/jamanetworkopen.2023.35813
Overall Survival Among Patients With De Novo Stage IV Metastatic and Distant Metastatic Recurrent Non-Small Cell Lung Cancer
Abstract
Importance: Despite recent breakthroughs in therapy, advanced lung cancer still poses a therapeutic challenge. The survival profile of patients with metastatic lung cancer remains poorly understood by metastatic disease type (ie, de novo stage IV vs distant recurrence).
Objective: To evaluate the association of metastatic disease type on overall survival (OS) among patients with non-small cell lung cancer (NSCLC) and to identify potential mechanisms underlying any survival difference.
Design, setting, and participants: Cohort study of a national US population based at a tertiary referral center in the San Francisco Bay Area using participant data from the National Lung Screening Trial (NLST) who were enrolled between 2002 and 2004 and followed up for up to 7 years as the primary cohort and patient data from Stanford Healthcare (SHC) for diagnoses between 2009 and 2019 and followed up for up to 13 years as the validation cohort. Participants from NLST with de novo metastatic or distant recurrent NSCLC diagnoses were included. Data were analyzed from January 2021 to March 2023.
Exposures: De novo stage IV vs distant recurrent metastatic disease.
Main outcomes and measures: OS after diagnosis of metastatic disease.
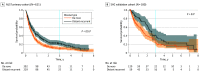
Results: The NLST and SHC cohort consisted of 660 and 180 participants, respectively (411 men [62.3%] vs 109 men [60.6%], 602 White participants [91.2%] vs 111 White participants [61.7%], and mean [SD] age of 66.8 [5.5] vs 71.4 [7.9] years at metastasis, respectively). Patients with distant recurrence showed significantly better OS than patients with de novo metastasis (adjusted hazard ratio [aHR], 0.72; 95% CI, 0.60-0.87; P < .001) in NLST, which was replicated in SHC (aHR, 0.64; 95% CI, 0.43-0.96; P = .03). In SHC, patients with de novo metastasis more frequently progressed to the bone (63 patients with de novo metastasis [52.5%] vs 19 patients with distant recurrence [31.7%]) or pleura (40 patients with de novo metastasis [33.3%] vs 8 patients with distant recurrence [13.3%]) than patients with distant recurrence and were primarily detected through symptoms (102 patients [85.0%]) as compared with posttreatment surveillance (47 patients [78.3%]) in the latter. The main finding remained consistent after further adjusting for metastasis sites and detection methods.
Conclusions and relevance: In this cohort study, patients with distant recurrent NSCLC had significantly better OS than those with de novo disease, and the latter group was associated with characteristics that may affect overall survival. This finding can help inform future clinical trial designs to ensure a balance for baseline patient characteristics.
Conflict of interest statement
Figures
References
-
- Mok TSK, Wu YL, Kudaba I, et al. ; KEYNOTE-042 Investigators . Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi:10.1016/S0140-6736(18)32409-7 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

