Dimethyl fumarate modulates the dystrophic disease program following short-term treatment
- PMID: 37751291
- PMCID: PMC10721277
- DOI: 10.1172/jci.insight.165974
Dimethyl fumarate modulates the dystrophic disease program following short-term treatment
Abstract
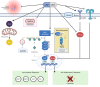
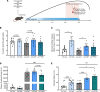
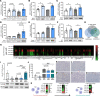
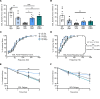
New medicines are urgently required to treat the fatal neuromuscular disease Duchenne muscular dystrophy (DMD). Dimethyl fumarate (DMF) is a potent immunomodulatory small molecule nuclear erythroid 2-related factor 2 activator with current clinical utility in the treatment of multiple sclerosis and psoriasis that could be effective for DMD and rapidly translatable. Here, we tested 2 weeks of daily 100 mg/kg DMF versus 5 mg/kg standard-care prednisone (PRED) treatment in juvenile mdx mice with early symptomatic DMD. Both drugs modulated seed genes driving the DMD disease program and improved force production in fast-twitch muscle. However, only DMF showed pro-mitochondrial effects, protected contracting muscles from fatigue, improved histopathology, and augmented clinically compatible muscle function tests. DMF may be a more selective modulator of the DMD disease program than PRED, warranting follow-up longitudinal studies to evaluate disease-modifying impact.
Keywords: Drug therapy; Muscle Biology; Neuromuscular disease; Skeletal muscle; Therapeutics.
Figures


References
-
- Ismail HM, et al. The potential and benefits of repurposing existing drugs to treat rare muscular dystrophies. Expert Opin Orphan Drugs. 2018;6(4):259–271. doi: 10.1080/21678707.2018.1452733. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

