Real-World Experience With Teprotumumab in Patients With Dysthyroid Optic Neuropathy
- PMID: 37751310
- PMCID: PMC10855992
- DOI: 10.1097/WNO.0000000000001994
Real-World Experience With Teprotumumab in Patients With Dysthyroid Optic Neuropathy
Abstract
Background: Teprotumumab, an insulin-like growth factor I receptor inhibitory antibody, improved proptosis, diplopia, inflammatory signs/symptoms, and quality of life in patients with active thyroid eye disease (TED) in clinical trials. The trials excluded patients with dysthyroid optic neuropathy (DON). Recently, many case reports and case series have reported the successful use of teprotumumab to treat DON. Here, we review the data from published cases and our clinical experience in treating patients having DON with teprotumumab.
Methods: A literature search was conducted of patients with DON treated with teprotumumab from January 2020 through September 2022. Data from DON patients from the authors' (M.A.T. and C.A.B.) clinical practice were included. Primary outcome measure was mean (SD) improvements for visual acuity, color vision, and visual fields. Improvements in proptosis and clinical activity score (CAS) and diplopia were compared before and after teprotumumab administration.
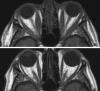
Results: Ten observational studies/case reports were identified along with 2 patients in our practice. In all, there were 24 active TED patients with DON (37 eyes) who were treated with teprotumumab. Mean (SD) age was 66.5 (13.6) years and 13 (54%) were females, disease duration ranged from 2 months to >15 years. 22/24 patients had none, minimal improvement or progression of visual loss with intravenous/oral corticosteroids, orbital decompression (n = 9), and orbital radiation (n = 2). There were 2 patients who received teprotumumab as the only therapy. Overall, 88% (21/24) reported improvement in visual acuity after teprotumumab and in 75% (18/24), improvement in vision was observed after just 2 infusions of teprotumumab. Three eyes had decompression surgery in close proximity to teprotumumab infusions and were excluded from analyses. Mean (SD) improvement in visual acuity was 3.73 lines (SD 3.74), range 2-15 lines in 33 eyes. The mean (SD) improvement in the mean deviation on visual field testing in 15 eyes was 5.6 db (3.0 db). Mean (SD) improvement in proptosis was 4.37 mm (SD: 2.11) (20 patients, 32 eyes); and clinical activity score: mean reduction of 5.1 (1.3) for 18 patients. Teprotumumab was well tolerated in all but one patient. Adverse events reported included fatigue, dysgeusia, hearing loss, nausea, hyperglycemia, and muscle spasms.
Conclusions: Teprotumumab is an effective treatment for DON in our experience and in published cases in whom treatment with steroids, surgery, or orbital radiation was unsuccessful.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the North American Neuro-Opthalmology Society.
Conflict of interest statement
M. A. Tamhankar and C. A. Briceño are consultants for Horizon Therapeutics. The remaining authors report no conflicts of interest.
Figures
References
-
- Douglas RS, Kahaly GJ, Patel A, et al. . Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382:341–352. - PubMed
-
- Lopez MJ, Herring JL, Thomas C, Bertram BA, Thomas DA. Visual recovery of dysthyroid optic neuropathy with teprotumumab. J Neuroophthalmol. 2022;42:e491–e493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous