Progress with induction of HIV broadly neutralizing antibodies in the Duke Consortia for HIV/AIDS Vaccine Development
- PMID: 37751363
- PMCID: PMC10552807
- DOI: 10.1097/COH.0000000000000820
Progress with induction of HIV broadly neutralizing antibodies in the Duke Consortia for HIV/AIDS Vaccine Development
Abstract
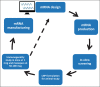
Purpose of review: Design of an HIV vaccine that can induce broadly neutralizing antibodies (bnAbs) is a major goal. However, HIV bnAbs are not readily made by the immune system. Rather HIV bnAbs are disfavored by a number of virus and host factors. The purpose of the review is to discuss recent progress made in the design and use of immunogens capable of inducing HIV bnAbs in the Duke Consortia for HIV/AIDS Vaccine Development.
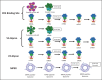
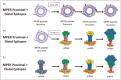
Recent findings: New immunogens capable of binding with high affinity to unmutated common ancestors (UCAs) of bnAb B cell lineages have been designed and strategies for stabilization of HIV Env in its prefusion state are being developed. Success is starting to be translated from preclinical studies of UCA-targeting immunogens in animals, to success of initiating bnAb lineages in humans.
Summary: Recent progress has been made in both immunogen design and in achieving bnAb B cell lineage induction in animal models and now in human clinical trials. With continued progress, a practical HIV/AIDS vaccine may be possible. However, host constraints on full bnAb maturation remain as potential roadblocks for full maturation of some types of bnAbs.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Figures

References
-
- Health NIo. HIV vaccine candidate does not sufficiently protect women against HIV infection. Available at: https://www.nih.gov/news-events/news-releases/hiv-vaccine-candidate-does....
-
- Health NIo. Experimental HIV vaccine regimen safe but ineffective, study finds. Available at: https://www.nih.gov/news-events/news-releases/experimental-hiv-vaccine-r....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

