Core-Labeling (Radio) Synthesis of Phenols
- PMID: 37751441
- PMCID: PMC10563162
- DOI: 10.1021/acs.orglett.3c02838
Core-Labeling (Radio) Synthesis of Phenols
Abstract
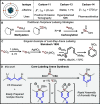
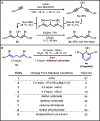
We report a method that enables the fast incorporation of carbon isotopes into the ipso carbon of phenols. Our approach relies on the synthesis of a 1,5-dibromo-1,4-pentadiene precursor, which upon lithium-halogen exchange followed by treatment with carbonate esters results in a formal [5 + 1] cyclization to form the phenol product. Using this strategy, we have prepared 12 1-13C-labeled phenols, show proof-of-concept for the labeling of phenols with carbon-14, and demonstrate phenol synthesis directly from cyclotron-produced [11C]CO2.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Synthesis of 13 C-labeled parabens from isotopically enriched phenols using the Houben-Hoesch reaction.J Labelled Comp Radiopharm. 2022 Jul;65(9):254-263. doi: 10.1002/jlcr.3992. Epub 2022 Jul 26. J Labelled Comp Radiopharm. 2022. PMID: 35868986
-
Direct Incorporation of [11C]CO2 into Asymmetric [11C]Carbonates.J Chem. 2018 Dec 10;2018:7641304. doi: 10.1155/2018/7641304. J Chem. 2018. PMID: 34079623 Free PMC article.
-
Ambident effect of a p-sulfinyl group for the introduction of two carbon substituents to phenol rings: a convergent synthesis of diverse benzofuran neolignans.Org Lett. 2000 Jul 27;2(15):2279-82. doi: 10.1021/ol0001261. Org Lett. 2000. PMID: 10930263
-
Catalytic properties of phenol carboxylase. In vitro study of CO2: 4-hydroxybenzoate isotope exchange reaction.Eur J Biochem. 1991 Apr 23;197(2):473-9. doi: 10.1111/j.1432-1033.1991.tb15934.x. Eur J Biochem. 1991. PMID: 1902788
-
[Efficient synthesis of multisubstituted aromatic compounds from phenol derivatives].Yakugaku Zasshi. 2014;134(12):1309-17. doi: 10.1248/yakushi.14-00192. Yakugaku Zasshi. 2014. PMID: 25452240 Review. Japanese.
Cited by
-
14N to 15N Isotopic Exchange of Nitrogen Heteroaromatics through Skeletal Editing.J Am Chem Soc. 2024 Feb 7;146(5):2950-2958. doi: 10.1021/jacs.3c11515. Epub 2024 Jan 29. J Am Chem Soc. 2024. PMID: 38286797 Free PMC article.
-
Pyridine-based strategies towards nitrogen isotope exchange and multiple isotope incorporation.Nat Commun. 2024 Jul 18;15(1):6063. doi: 10.1038/s41467-024-50139-w. Nat Commun. 2024. PMID: 39025881 Free PMC article.
-
Strategic atom replacement enables regiocontrol in pyrazole alkylation.Nature. 2025 May;641(8063):646-652. doi: 10.1038/s41586-025-08951-x. Epub 2025 Apr 3. Nature. 2025. PMID: 40179959
References
Grants and funding
LinkOut - more resources
Full Text Sources

