Comparing methods to classify admitted patients with SARS-CoV-2 as admitted for COVID-19 versus with incidental SARS-CoV-2: A cohort study
- PMID: 37751455
- PMCID: PMC10522023
- DOI: 10.1371/journal.pone.0291580
Comparing methods to classify admitted patients with SARS-CoV-2 as admitted for COVID-19 versus with incidental SARS-CoV-2: A cohort study
Erratum in
-
Correction: Comparing methods to classify admitted patients with SARS-CoV-2 as admitted for COVID-19 versus with incidental SARS-CoV-2: A cohort study.PLoS One. 2025 Mar 18;20(3):e0320617. doi: 10.1371/journal.pone.0320617. eCollection 2025. PLoS One. 2025. PMID: 40100805 Free PMC article.
Abstract
Introduction: Not all patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection develop symptomatic coronavirus disease 2019 (COVID-19), making it challenging to assess the burden of COVID-19-related hospitalizations and mortality. We aimed to determine the proportion, resource utilization, and outcomes of SARS-CoV-2 positive patients admitted for COVID-19, and assess the impact of using the Center for Disease Control's (CDC) discharge diagnosis-based algorithm and the Massachusetts state department's drug administration-based classification system on identifying admissions for COVID-19.
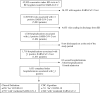
Methods: In this retrospective cohort study, we enrolled consecutive SARS-CoV-2 positive patients admitted to one of five hospitals in British Columbia between December 19, 2021 and May 31,2022. We completed medical record reviews, and classified hospitalizations as being primarily for COVID-19 or with incidental SARS-CoV-2 infection. We applied the CDC algorithm and the Massachusetts classification to estimate the difference in hospital days, intensive care unit (ICU) days and in-hospital mortality and calculated sensitivity and specificity.
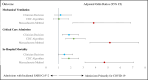
Results: Of 42,505 Emergency Department patients, 1,651 were admitted and tested positive for SARS-CoV-2, with 858 (52.0%, 95% CI 49.6-54.4) admitted for COVID-19. Patients hospitalized for COVID-19 required ICU admission (14.0% versus 8.2%, p<0.001) and died (12.6% versus 6.4%, p<0.001) more frequently compared with patients with incidental SARS-CoV-2. Compared to case classification by clinicians, the CDC algorithm had a sensitivity of 82.9% (711/858, 95% CI 80.3%, 85.4%) and specificity of 98.1% (778/793, 95% CI 97.2%, 99.1%) for COVID-19-related admissions and underestimated COVID-19 attributable hospital days. The Massachusetts classification had a sensitivity of 60.5% (519/858, 95% CI 57.2%, 63.8%) and specificity of 78.6% (623/793, 95% CI 75.7%, 81.4%) for COVID-19-related admissions, underestimating total number of hospital and ICU bed days while overestimating COVID-19-related intubations, ICU admissions, and deaths.
Conclusion: Half of SARS-CoV-2 hospitalizations were for COVID-19 during the Omicron wave. The CDC algorithm was more specific and sensitive than the Massachusetts classification, but underestimated the burden of COVID-19 admissions.
Trial registration: Clinicaltrials.gov, NCT04702945.
Copyright: © 2023 Hohl et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Drs. Perry and Atzema have peer reviewed mid-career salary support awards from the Heart and Stroke Foundation of Ontario. Dr. Hohl is supported by a Michael Smith Foundation Health Professional Investigator Award. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Health Canada. COVID-19 wastewater surveillance dashboard. 27 Sep 2022. [cited 3 Oct 2022]. Available: https://health-infobase.canada.ca/covid-19/wastewater/
-
- Fillmore NR, La J, Zheng C, Doron S, Do NV, Monach PA, et al.. The COVID-19 hospitalization metric in the pre- and postvaccination eras as a measure of pandemic severity: A retrospective, nationwide cohort study. Infect Control Hosp Epidemiol. 2022; 1–6. doi: 10.1017/ice.2022.13 - DOI - PMC - PubMed
-
- Català M, Coma E, Alonso S, Andrés C, Blanco I, Antón A, et al.. Transmissibility, hospitalization, and intensive care admissions due to omicron compared to delta variants of SARS-CoV-2 in Catalonia: A cohort study and ecological analysis. Front Public Health. 2022;10: 961030. doi: 10.3389/fpubh.2022.961030 - DOI - PMC - PubMed
-
- Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, et al.. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399: 1618–1624. doi: 10.1016/S0140-6736(22)00327-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

