The Best Models of Bodipy's Electronic Excited State: Comparing Predictions from Various DFT Functionals with Measurements from Femtosecond Stimulated Raman Spectroscopy
- PMID: 37751471
- PMCID: PMC10561280
- DOI: 10.1021/acs.jpca.3c05040
The Best Models of Bodipy's Electronic Excited State: Comparing Predictions from Various DFT Functionals with Measurements from Femtosecond Stimulated Raman Spectroscopy
Abstract
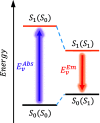
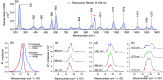
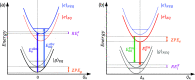
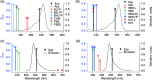
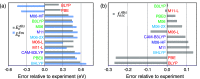
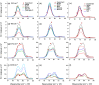
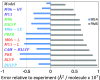
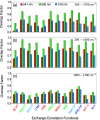
Density functional theory (DFT) and time-dependent DFT (TD-DFT) are pivotal approaches for modeling electronically excited states of molecules. However, choosing a DFT exchange-correlation functional (XCF) among the myriad of alternatives is an overwhelming task that can affect the interpretation of results and lead to erroneous conclusions. The performance of these XCFs to describe the excited-state properties is often addressed by comparing them with high-level wave function methods or experimentally available vertical excitation energies; however, this is a limited analysis that relies on evaluation of a single point in the excited-state potential energy surface (PES). Different strategies have been proposed but are limited by the difficulty of experimentally accessing the electronic excited-state properties. In this work, we have tested the performance of 12 different XCFs and TD-DFT to describe the excited-state potential energy surface of Bodipy (2,6-diethyl-1,3,5,7-tetramethyl-8-phenyldipyrromethene difluoroborate). We compare those results with resonance Raman spectra collected by using femtosecond stimulated Raman spectroscopy (FSRS). By simultaneously fitting the absorption spectrum, fluorescence spectrum, and all of the resonance Raman excitation profiles within the independent mode displaced harmonic oscillator (IMDHO) formalism, we can describe the PES at the Franck-Condon (FC) region and determine the solvent and intramolecular reorganization energy after relaxation. This allows a direct comparison of the TD-DFT output with experimental observables. Our analysis reveals that using vertical absorption energies might not be a good criterion to determine the best XCF for a given molecular system and that FSRS opens up a new way to benchmark the excited-state performance of XCFs of fluorescent dyes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Hohenberg P.; Kohn W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. 10.1103/PhysRev.136.B864. - DOI
-
- Kohn W.; Sham L. J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. 10.1103/PhysRev.140.A1133. - DOI
-
- Cramer C. J.Essentials of Computational Chemistry: Theories and Models, 2nd ed.; John Wiley & Sons, 2004; p 257.
-
- McQuarrie D. A.Quantum Chemistry, 2nd ed.; University Science Books, 2007; pp 445, 454.
LinkOut - more resources
Full Text Sources

