α-lipoic acid ameliorates consequences of copper overload by up-regulating selenoproteins and decreasing redox misbalance
- PMID: 37751556
- PMCID: PMC10556618
- DOI: 10.1073/pnas.2305961120
α-lipoic acid ameliorates consequences of copper overload by up-regulating selenoproteins and decreasing redox misbalance
Abstract
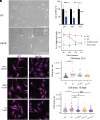
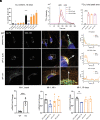
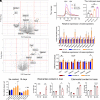
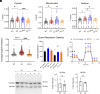
α-lipoic acid (LA) is an essential cofactor for mitochondrial dehydrogenases and is required for cell growth, metabolic fuel production, and antioxidant defense. In vitro, LA binds copper (Cu) with high affinity and as an endogenous membrane permeable metabolite could be advantageous in mitigating the consequences of Cu overload in human diseases. We tested this hypothesis in 3T3-L1 preadipocytes with inactivated Cu transporter Atp7a; these cells accumulate Cu and show morphologic changes and mitochondria impairment. Treatment with LA corrected the morphology of Atp7a-/- cells similar to the Cu chelator bathocuproinedisulfonate (BCS) and improved mitochondria function; however, the mechanisms of LA and BCS action were different. Unlike BCS, LA did not decrease intracellular Cu but instead increased selenium levels that were low in Atp7a-/- cells. Proteome analysis confirmed distinct cell responses to these compounds and identified upregulation of selenoproteins as the major effect of LA on preadipocytes. Upregulation of selenoproteins was associated with an improved GSH:GSSG ratio in cellular compartments, which was lowered by elevated Cu, and reversal of protein oxidation. Thus, LA diminishes toxic effects of elevated Cu by improving cellular redox environment. We also show that selenium levels are decreased in tissues of a Wilson disease animal model, especially in the liver, making LA an attractive candidate for supplemental treatment of this disease.
Keywords: Wilson disease; copper; oxidative stress; selenoprotein; α-lipoic acid.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
R-alpha-lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes.Arch Biochem Biophys. 2002 Jan 15;397(2):384-91. doi: 10.1006/abbi.2001.2680. Arch Biochem Biophys. 2002. PMID: 11795898
-
Selenium homeostasis in human brain cells: Effects of copper (II) and Se species.J Trace Elem Med Biol. 2023 Jul;78:127149. doi: 10.1016/j.jtemb.2023.127149. Epub 2023 Mar 12. J Trace Elem Med Biol. 2023. PMID: 36948045
-
Redox regulation of mitochondrial permeability transition: effects of uncoupler, lipoic acid and its positively charged analog LA-plus and selenium.Biofactors. 2003;17(1-4):297-306. doi: 10.1002/biof.5520170129. Biofactors. 2003. PMID: 12897451 Review. No abstract available.
-
The antioxidant effect of DL-alpha-lipoic acid on copper-induced acute hepatitis in Long-Evans Cinnamon (LEC) rats.Free Radic Res. 2001 Jan;34(1):69-80. doi: 10.1080/10715760100300071. Free Radic Res. 2001. PMID: 11234997
-
Selenium, a Micronutrient That Modulates Cardiovascular Health via Redox Enzymology.Nutrients. 2021 Sep 17;13(9):3238. doi: 10.3390/nu13093238. Nutrients. 2021. PMID: 34579115 Free PMC article. Review.
Cited by
-
Exploring the Efficacy of Alpha-Lipoic Acid in Comorbid Osteoarthritis and Type 2 Diabetes Mellitus.Nutrients. 2024 Oct 2;16(19):3349. doi: 10.3390/nu16193349. Nutrients. 2024. PMID: 39408316 Free PMC article.
-
Mammalian copper homeostasis: physiological roles and molecular mechanisms.Physiol Rev. 2025 Jan 1;105(1):441-491. doi: 10.1152/physrev.00011.2024. Epub 2024 Aug 22. Physiol Rev. 2025. PMID: 39172219 Free PMC article. Review.
-
Alpha-Lipoic Acid: Biological Mechanisms and Health Benefits.Antioxidants (Basel). 2024 Oct 12;13(10):1228. doi: 10.3390/antiox13101228. Antioxidants (Basel). 2024. PMID: 39456481 Free PMC article. Review.
-
Nanoparticle-Driven Skeletal Muscle Repair and Regeneration Through Macrophage-Muscle Stem Cell Interaction.Small. 2025 May;21(18):e2412611. doi: 10.1002/smll.202412611. Epub 2025 Mar 20. Small. 2025. PMID: 40109124 Free PMC article.
-
Copper metabolism in osteoarthritis and its relation to oxidative stress and ferroptosis in chondrocytes.Front Mol Biosci. 2024 Sep 11;11:1472492. doi: 10.3389/fmolb.2024.1472492. eCollection 2024. Front Mol Biosci. 2024. PMID: 39329090 Free PMC article. Review.
References
-
- Uriu-Adams J. Y., Keen C. L., Copper, oxidative stress, and human health. Mol. Aspects Med. 26, 268–298 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

