Electrochemical Late-Stage Functionalization
- PMID: 37751573
- PMCID: PMC10571048
- DOI: 10.1021/acs.chemrev.3c00158
Electrochemical Late-Stage Functionalization
Abstract
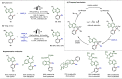
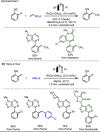
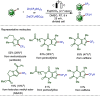
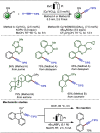
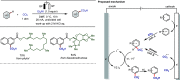
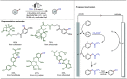
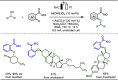
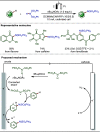
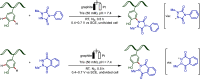
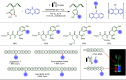
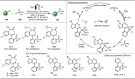
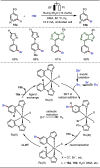
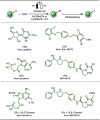
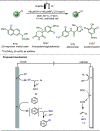
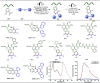
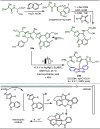
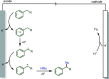
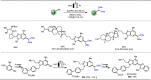
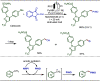
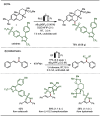
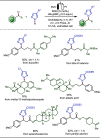
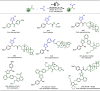
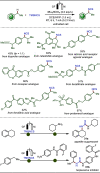
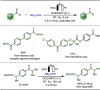
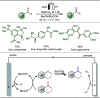
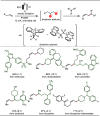
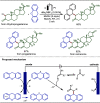
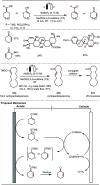
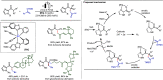
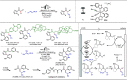
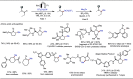
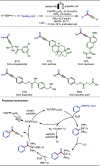
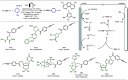
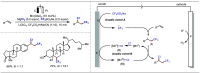
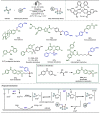
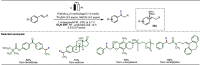
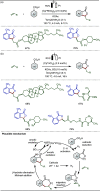
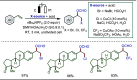
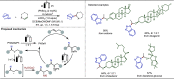
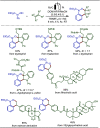
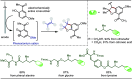
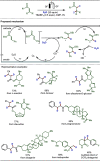
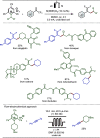
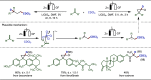
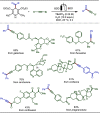
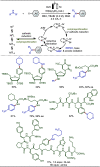
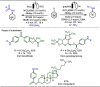
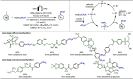
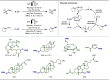
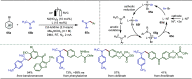
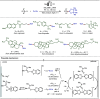
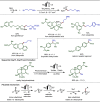
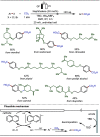
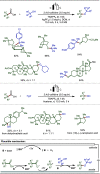
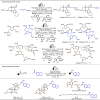
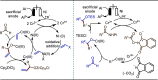
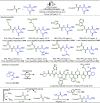
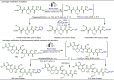
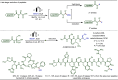
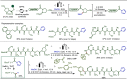
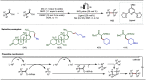
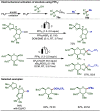
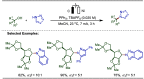
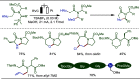
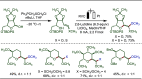
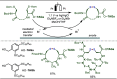
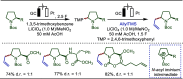
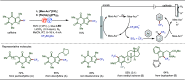
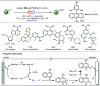
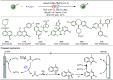
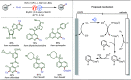
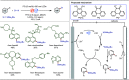
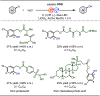
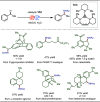
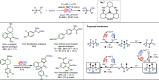
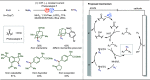
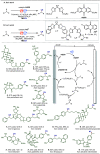
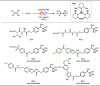
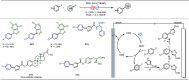
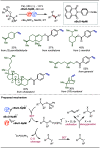
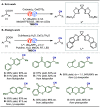
Late-stage functionalization (LSF) constitutes a powerful strategy for the assembly or diversification of novel molecular entities with improved physicochemical or biological activities. LSF can thus greatly accelerate the development of medicinally relevant compounds, crop protecting agents, and functional materials. Electrochemical molecular synthesis has emerged as an environmentally friendly platform for the transformation of organic compounds. Over the past decade, electrochemical late-stage functionalization (eLSF) has gained major momentum, which is summarized herein up to February 2023.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


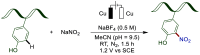
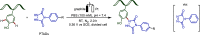
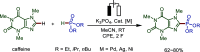

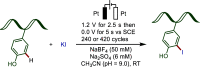









References
-
- Dominguez-Huerta A.; Dai X.-J.; Zhou F.; Querard P.; Qiu Z.; Ung S.; Liu W.; Li J.; Li C.-J. Exploration of New Reaction Tools for Late-Stage Functionalization of Complex Chemicals. Can. J. Chem. 2019, 97, 67–85. 10.1139/cjc-2018-0357. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

