The oleic/palmitic acid imbalance in exosomes isolated from NAFLD patients induces necroptosis of liver cells via the elongase-6/RIP-1 pathway
- PMID: 37752143
- PMCID: PMC10522611
- DOI: 10.1038/s41419-023-06161-9
The oleic/palmitic acid imbalance in exosomes isolated from NAFLD patients induces necroptosis of liver cells via the elongase-6/RIP-1 pathway
Abstract
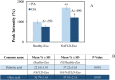
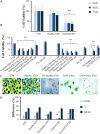
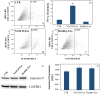
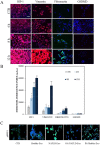
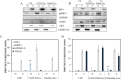
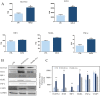
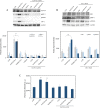
Excessive toxic lipid accumulation in hepatocytes underlies the development of non-alcoholic fatty liver disease (NAFLD), phenotypically characterized by necrosis and steato-fibrosis, whose molecular mechanism is not yet fully understood. Patients with NAFLD display an imbalanced palmitic (PA) to oleic acid (OA) ratio. Moreover, increasing experimental evidence points out a relevant involvement of the exosomal content in disease progression. Aim of the study was to highlight the PA/OA imbalance within circulating exosomes, the subsequent intracellular alterations, and the impact on NALFD. Liver cells were challenged with exosomes isolated from both healthy subjects and NAFLD patients. The exosomal PA/OA ratio was artificially modified, and biological effects were evaluated. A NAFLD-derived exosomal PA/OA imbalance impacts liver cell cycle and cell viability. OA-modified NAFLD-derived exosomes restored cellular viability and proliferation, whereas the inclusion of PA into healthy subjects-derived exosomes negatively affected cell viability. Moreover, while OA reduced the phosphorylation and activation of the necroptosis marker, Receptor-interacting protein 1 (phospho-RIP-1), PA induced the opposite outcome, alongside increased levels of stress fibers, such as vimentin and fibronectin. Administration of NAFLD-derived exosomes led to increased expression of Elongase 6 (ELOVL6), Stearoyl-CoA desaturase 1 (SCD1), Tumor necrosis factor α (TNF-α), Mixed-lineage-kinase-domain-like-protein (MLKL) and RIP-1 in the hepatocytes, comparable to mRNA levels in the hepatocytes of NAFLD patients reported in the Gene Expression Omnibus (GEO) database. Genetic and pharmacological abrogation of ELOVL6 elicited a reduced expression of downstream molecules TNF-α, phospho-RIP-1, and phospho-MLKL upon administration of NAFLD-derived exosomes. Lastly, mice fed with high-fat diet exhibited higher phospho-RIP-1 than mice fed with control diet. Targeting the Elongase 6-RIP-1 signaling pathway offers a novel therapeutic approach for the treatment of the NALFD-induced exosomal PA/OA imbalance.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

