Purification and characterization of cysteine protease of Sarcocystis fusiformis from infected Egyptian water buffaloes
- PMID: 37752241
- PMCID: PMC10522634
- DOI: 10.1038/s41598-023-43147-1
Purification and characterization of cysteine protease of Sarcocystis fusiformis from infected Egyptian water buffaloes
Abstract
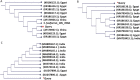
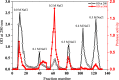
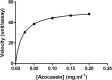
Sarcocystis spp. infects water buffaloes (Bubalus bubalis) causing sarcocystosis. In the present study, Sarcocystis fusiformis was recognized in Egyptian water buffaloes based on histological observation and molecular analysis of internal transcribed spacer 1 (ITS1), 18S ribosomal RNA (18S rRNA) and cytochrome c oxidase subunit I (COX-1) gene fragments. Chemotherapy and vaccines against Sarcocystis spp. could potentially target proteases because they may play a crucial role in the infection. Cysteine proteases are multifunctional enzymes involved in vital metabolic processes. However, the involvement of proteases in S. fusiform infection has not yet been characterized. Here, the purification and study on some biochemical properties of protease isolated from cysts of S. fusiform were carried out. Protease with a molecular weight of 100 kDa was purified. LC-MS/MS analyzed the protein sequence of purified protease and the data suggested that the enzyme might be related to the cysteine protease. The purified protease exhibited maximum activity at pH 6 and a temperature of 50 °C. The Michaelis-Menten constant (Km), the maximum velocity (Vmax), and the turnover number (Kcat) were determined. The complete inhibition effect of cysteine inhibitors indicated that the purified enzyme is a cysteine protease. The results suggested that S. fusiform proteolytic enzyme may be necessary for parasite survival in water buffaloes by digesting host tissues. Therefore, cysteine protease could be a suitable target for vaccinations.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Molecular differentiation of Sarcocystis buffalonis and Sarcocystis levinei in water buffaloes (Bubalus bubalis) from Sarcocystis hirsuta and Sarcocystis cruzi in cattle (Bos taurus).Parasitol Res. 2016 Jun;115(6):2459-71. doi: 10.1007/s00436-016-4998-1. Epub 2016 Mar 16. Parasitol Res. 2016. PMID: 26979729
-
Molecular characterisation of three regions of the nuclear ribosomal DNA unit and the mitochondrial cox1 gene of Sarcocystis fusiformis from water buffaloes (Bubalus bubalis) in Egypt.Parasitol Res. 2015 Sep;114(9):3401-13. doi: 10.1007/s00436-015-4566-0. Epub 2015 Jun 9. Parasitol Res. 2015. PMID: 26051128
-
Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis).Parasitol Res. 2016 Apr;115(4):1473-92. doi: 10.1007/s00436-015-4881-5. Epub 2015 Dec 17. Parasitol Res. 2016. PMID: 26677095
-
Identity of Sarcocystis species of the water buffalo (Bubalus bubalis) and cattle (Bos taurus) and the suppression of Sarcocystis sinensis as a nomen nudum.Vet Parasitol. 2014 Sep 15;205(1-2):1-6. doi: 10.1016/j.vetpar.2014.06.020. Epub 2014 Jun 19. Vet Parasitol. 2014. PMID: 25034134 Review.
-
The resurrection of a species: Sarcocystis bovifelis Heydorn et al., 1975 is distinct from the current Sarcocystis hirsuta in cattle and morphologically indistinguishable from Sarcocystis sinensis in water buffaloes.Parasitol Res. 2016 Jan;115(1):1-21. doi: 10.1007/s00436-015-4785-4. Epub 2015 Oct 14. Parasitol Res. 2016. PMID: 26462803 Review.
Cited by
-
Sarcocystis species: molecular identification and seroprevalence in water buffaloes (Bubalus bubalis).BMC Vet Res. 2025 Jul 22;21(1):482. doi: 10.1186/s12917-025-04933-3. BMC Vet Res. 2025. PMID: 40696429 Free PMC article.
References
-
- Daryani A, Alaei R, Dehghan M, Arab R, Sharif M, Ziaei H. Survey of Sarcocystis infection in slaughtered sheep and buffaloes in Ardabil, Iran. J. Anim. Vet. Adv. 2006;5:60–62.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

