Formulation development, in vivo bioequivalence and pediatric PBPK modeling studies of taste-masked ciprofloxacin chewable tablets
- PMID: 37752265
- PMCID: PMC10522605
- DOI: 10.1038/s41598-023-43423-0
Formulation development, in vivo bioequivalence and pediatric PBPK modeling studies of taste-masked ciprofloxacin chewable tablets
Abstract
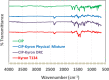
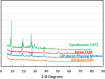
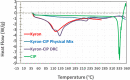
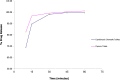
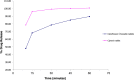
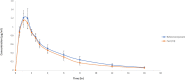
A taste-masked chewable tablet of ciprofloxacin using ion exchange resin Kyron T-134 for enhancing compliance for the paediatric population was developed. The drug-to-resin ratio was optimized for maximum taste masking by studying the effects of soaking time (X1) and mixing time (X2) on complexation (%) using Central Composite Rotatable Design (CCRD). The resin complexes were characterized by bitterness score, DSC, FTIR, and PXRD. The complex was further formulated and optimized into chewable tablets through full factorial design, The optimized formulation was subjected to a bioequivalence study, and a virtual approach of PBPK modelling was adapted to predict the pharmacokinetics of the drug in the paediatric group. The drug resin ratio of 1:1.5 yielded an optimum drug loading of 99.05%. The optimized formulation shows minimum disintegration time with more than 99% drug release within 30 min. The formulation F-9 was found to be bioequivalent with a geometric mean ratio of Cmax, Tmax, AUC0-t, and AUC0-∞ within 90% CI. It was concluded that quality by design approach can successfully be applied to optimize the drug resin ratio and PBPK modeling is a successful predictive tool for estimating the pharmacokinetics of ciprofloxacin HCl in the paediatric population.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Gao, R., Shao, Z. J., Fan, A. C.-L., Witchey-Lakshmanan, L. C. & Stewart, D. C. (Google Patents, 2003).
-
- Long, C. Biochemists' Handbook (1961).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

