Long-term utilization and benefit of luspatercept in transfusion-dependent, erythropoiesis-stimulating agent-refractory or -intolerant patients with lower-risk myelodysplastic syndromes with ring sideroblasts
- PMID: 37752285
- PMCID: PMC10624606
- DOI: 10.1038/s41375-023-02031-7
Long-term utilization and benefit of luspatercept in transfusion-dependent, erythropoiesis-stimulating agent-refractory or -intolerant patients with lower-risk myelodysplastic syndromes with ring sideroblasts
Conflict of interest statement
UP has received grant support from Amgen, Janssen, Merck, and Novartis; grant and travel support, and lecture, steering committee, and consulting fees, and travel support from Celgene/BMS; and lecture fees from Novartis. VS has received honoraria and advisory board fees from BMS, travel support from Janssen, and advisory board fees from AbbVie, Geron, Gilead, Menarini, Novartis, Servier Pharmaceuticals, and Syros. RSK has received consulting and advisory board fees from Geron; speaker and advisory board fees from AbbVie, CTI BioPharma, Jazz Pharmaceuticals, PharmaEssentia, and Servier Pharmaceuticals; advisory board fees from Novartis, Rigel Pharmaceuticals, and Taiho Pharma; and funding and advisory board fees from BMS. AMZ has received consulting fees, research funding, honoraria, and travel support from Cardiff Oncology, Novartis, and Pfizer; consulting fees, research funding, and honoraria from AbbVie, Amgen, Aprea, Boehringer-Ingelheim, Celgene/BMS, Incyte, Otsuka, Takeda, and Trovagene; consulting fees, and honoraria from Acceleron Pharma, Agios, Astellas, BeyondSpring, Cardinal Health, Daiichi Sankyo, Epizyme, Geron, Gilead, Ionis Pharmaceuticals, Janssen, Jazz Pharmaceuticals, Kura Oncology, Seattle Genetics, Syndax, Taiho Pharma, Tyme; research funding from ADC Therapeutics, Astex Pharmaceuticals, AstraZeneca, and Medimmune. GG-M has received research funding from BMS. RB has received research funding and honoraria from BMS, and Taiho Pharma, and honoraria from AbbVie. DM, NH, BR, and AY are currently employed by BMS. JAN is currently employed by BMS, is a current holder of individual stocks in a privately held company, and a current holder of BMS stock options. KK is currently employed by, is a current holder of BMS stock options, and is a current holder of Pfizer stock options. YL, ACG, and SV are currently employed by, and current holders of BMS stock options. PF has received consulting fees, research funding, and honoraria from BMS.
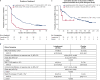
Figures

References
-
- Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica. 2006;91:1588–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

