Hepatocyte-specific HDAC3 ablation promotes hepatocellular carcinoma in females by suppressing Foxa1/2
- PMID: 37752418
- PMCID: PMC10521566
- DOI: 10.1186/s12885-023-11393-1
Hepatocyte-specific HDAC3 ablation promotes hepatocellular carcinoma in females by suppressing Foxa1/2
Abstract
Background: Hepatocellular carcinoma (HCC), the most common primary liver cancer, prevails mainly in males and has long been attributed to androgens and higher circumstantial levels of interleukin-6 (IL-6) produced by resident hepatic macrophages.
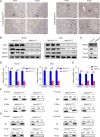
Methods: Constitutively hepatocyte-specific histone deacetylase 3 (HDAC3)-deficient (HDAC3LCKO) mice and constitutively hepatocyte-specific HDAC3 knockout and systemic IL-6 simultaneously ablated (HDAC3LCKO& IL-6-/-) mice were used in our study to explore the causes of sex differences in HCC. Additionally, we performed human HCC tissues with an IHC score. Correlation analysis and linear regression plots were constructed to reveal the association between HDAC3 and its candidate genes. To further elucidate that HDAC3 controls the expression of Foxa1/2, we knocked down HDAC3 in HUH7 liver cancer cells.
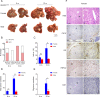
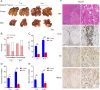
Results: We observed a contrary sex disparity, with an earlier onset and higher incidence of HCC in female mice when HDAC3 was selectively ablated in the liver. Loss of HDAC3 led to constant liver injury and the spontaneous development of HCC. Unlike the significant elevation of IL-6 in male mice at a very early age, female mice exhibit stable IL-6 levels, and IL-6 ablation did not eliminate the sex disparity in hepatocarcinogenesis in HDAC3-deficient mice. Oestrogen often protects the liver when combined with oestrogen receptor alpha (ERα); however, ovariectomy in HDAC3-ablated female mice significantly delayed tumourigenesis. The oestrogen-ERα axis can also play a role in tumour promotion in the absence of Foxa1 and Foxa2 in the receptor complex. Loss of HDAC3 profoundly reduced the expression of both Foxa1 and Foxa2 and impaired the binding between Foxa1/2 and ERα. Furthermore, a more frequent HDAC3 decrease accompanied by the simultaneous Foxa1/2 decline was found in female HCC compared to that in male HCC.
Conclusion: In summary, we reported that loss of HDAC3 reduces Foxa1/2 and thus promotes HCC development in females in an oestrogen-dependent manner.
Keywords: Foxa1; Foxa2; HCC; HDAC3; IL-6; Oestrogen receptor; Sex difference.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no conflict of Interests.
Figures




References
-
- Thomas L, Petrick J, McGlynn K. Liver cancer. In: Thun M, Linet M, Cerhan J, Haiman C, Schottenfeld D, editors. Cancer Epidemiology and Prevention. 4th ed. Oxford University Press; 2018. pp. 635–60.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

