Effects of tail nerve electrical stimulation on the activation and plasticity of the lumbar locomotor circuits and the prevention of skeletal muscle atrophy after spinal cord transection in rats
- PMID: 37752787
- PMCID: PMC10916423
- DOI: 10.1111/cns.14445
Effects of tail nerve electrical stimulation on the activation and plasticity of the lumbar locomotor circuits and the prevention of skeletal muscle atrophy after spinal cord transection in rats
Abstract
Introduction: Severe spinal cord injury results in the loss of neurons in the relatively intact spinal cord below the injury area and skeletal muscle atrophy in the paralyzed limbs. These pathological processes are significant obstacles for motor function reconstruction.
Objective: We performed tail nerve electrical stimulation (TNES) to activate the motor neural circuits below the injury site of the spinal cord to elucidate the regulatory mechanisms of the excitatory afferent neurons in promoting the reconstruction of locomotor function.
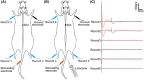
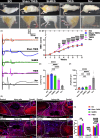
Methods: Eight days after T10 spinal cord transection in rats, TNES was performed for 7 weeks. Behavioral scores were assessed weekly. Electrophysiological tests and double retrograde tracings were performed at week 8.
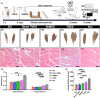
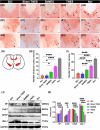
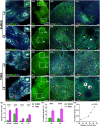
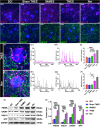
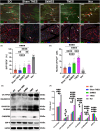
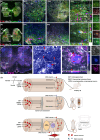
Results: After 7 weeks of TNES treatment, there was restoration in innervation, the number of stem cells, and mitochondrial metabolism in the rats' hindlimb muscles. Double retrograde tracings of the tail nerve and sciatic nerve further confirmed the presence of synaptic connections between the tail nerve and central pattern generator (CPG) neurons in the lumbar spinal cord, as well as motor neurons innervating the hindlimb muscles.
Conclusion: The mechanisms of TNES induced by the stimulation of primary afferent nerve fibers involves efficient activation of the motor neural circuits in the lumbosacral segment, alterations of synaptic plasticity, and the improvement of muscle and nerve regeneration, which provides the structural and functional foundation for the future use of cutting-edge biological treatment strategies to restore voluntary movement of paralyzed hindlimbs.
Keywords: motor neural circuit activation; muscle atrophy; regeneration; spinal cord transection; tail nerve electrical stimulation.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest. Animals were used with the approval of the ethics committee of Sun Yat‐sen University (Animal Use Protocol no. SYSU‐IACUC‐2019‐B1101).
Figures
Similar articles
-
Tail nerve electrical stimulation promoted the efficiency of transplanted spinal cord-like tissue as a neuronal relay to repair the motor function of rats with transected spinal cord injury.Biomaterials. 2023 Jun;297:122103. doi: 10.1016/j.biomaterials.2023.122103. Epub 2023 Mar 28. Biomaterials. 2023. PMID: 37028111
-
Tail Nerve Electrical Stimulation and Electro-Acupuncture Can Protect Spinal Motor Neurons and Alleviate Muscle Atrophy after Spinal Cord Transection in Rats.Neural Plast. 2017;2017:7351238. doi: 10.1155/2017/7351238. Epub 2017 Jun 28. Neural Plast. 2017. PMID: 28744378 Free PMC article.
-
The therapeutic mechanism of transcranial iTBS on nerve regeneration and functional recovery in rats with complete spinal cord transection.Front Immunol. 2023 Jun 14;14:1153516. doi: 10.3389/fimmu.2023.1153516. eCollection 2023. Front Immunol. 2023. PMID: 37388732 Free PMC article.
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
-
Probing the Human Spinal Locomotor Circuits by Phasic Step-Induced Feedback and by Tonic Electrical and Pharmacological Neuromodulation.Curr Pharm Des. 2017;23(12):1805-1820. doi: 10.2174/1381612822666161214144655. Curr Pharm Des. 2017. PMID: 27981912 Review.
Cited by
-
Central Pattern Generators in Spinal Cord Injury: Mechanisms, Modulation, and Therapeutic Strategies for Motor Recovery.JOR Spine. 2025 Aug 11;8(3):e70100. doi: 10.1002/jsp2.70100. eCollection 2025 Sep. JOR Spine. 2025. PMID: 40791879 Free PMC article. Review.
-
Self-Healing COCu-Tac Hydrogel Enhances iNSCs Transplantation for Spinal Cord Injury by Promoting Mitophagy via the FKBP52/AKT Pathway.Adv Sci (Weinh). 2025 Jan;12(3):e2407757. doi: 10.1002/advs.202407757. Epub 2024 Nov 25. Adv Sci (Weinh). 2025. PMID: 39587837 Free PMC article.
-
DON-Apt19S bioactive scaffold transplantation promotes in situ spinal cord repair in rats with transected spinal cord injury by effectively recruiting endogenous neural stem cells and mesenchymal stem cells.Mater Today Bio. 2025 Apr 10;32:101753. doi: 10.1016/j.mtbio.2025.101753. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40275960 Free PMC article.
-
Multi-target neural circuit reconstruction and enhancement in spinal cord injury.Neural Regen Res. 2026 Mar 1;21(3):957-971. doi: 10.4103/NRR.NRR-D-24-00434. Epub 2025 Jan 29. Neural Regen Res. 2026. PMID: 39885668 Free PMC article.
-
SGK1 upregulation in GFAP+ neurons in the frontal association cortex protects against neuronal apoptosis after spinal cord injury.Cell Death Dis. 2025 Apr 2;16(1):237. doi: 10.1038/s41419-025-07542-y. Cell Death Dis. 2025. PMID: 40175324 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

