A novel autosomal dominant ERLIN2 variant activates endoplasmic reticulum stress in a Chinese HSP family
- PMID: 37752894
- PMCID: PMC10646992
- DOI: 10.1002/acn3.51902
A novel autosomal dominant ERLIN2 variant activates endoplasmic reticulum stress in a Chinese HSP family
Abstract
Objective: Hereditary spastic paraplegia (HSP) has been reported rarely because of a monoallelic variant in ERLIN2. The present study aimed at describing a novel autosomal dominant ERLIN2 pedigree in a Chinese family and exploring the possible mechanism of HSP caused by ERLIN2 variants.
Methods: The proband and his family underwent a comprehensive medical history inquiry and neurological examinations. Whole-exome sequencing was performed on the proband, and Sanger sequencing was performed on some family members. HeLa cell lines and mouse primary cortical neurons were used for immunofluorescence (IF) and reverse transcription-PCR (RT-PCR).
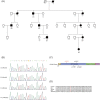
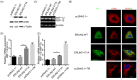
Results: Seven patients were clinically diagnosed with pure spastic paraplegia in four consecutive generations with the autosomal dominant inheritance model. All patients presented juvenile-adolescent onset and gradually worsening pure HSP phenotype. Whole-exome sequencing of the proband and Sanger sequencing of all available family members identified a novel heterozygous c.212 T>C (p.V71A) variant in exon 8 of the ERLIN2 gene. The c.212 T>C demonstrated a high pathogenic effect score through functional prediction. RT-PCR and IF analysis of overexpressed V71A revealed an altered ER morphology and increased XBP-1S mRNA levels, suggesting the activation of ER stress. Overexpression of V71A in primary cultured cortical neurons promoted axon growth.
Interpretation: The novel c.212 T>C heterozygous variant in human ERLIN2 caused pure HSP. Moreover, c.212 T>C heterozygous variant in ERLIN2 increased ER stress and affected axonal development.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors state that there is no conflict of interest.
Figures

References
-
- Salinas S, Proukakis C, Crosby A, Warner TT. Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol. 2008;7(12):1127‐1138. - PubMed
-
- Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42(3):174‐183. - PubMed
-
- Coutinho P, Ruano L, Loureiro JL, et al. Hereditary ataxia and spastic paraplegia in Portugal: a population‐based prevalence study. JAMA Neurol. 2013;70(6):746‐755. - PubMed
-
- Harding A. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1(8334):1151‐1155. - PubMed
-
- Shribman S, Reid E, Crosby AH, Houlden H, Warner TT. Hereditary spastic paraplegia: from diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019;18(12):1136‐1146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

