Effect of unguided e-cigarette provision on uptake, use, and smoking cessation among adults who smoke in the USA: a naturalistic, randomised, controlled clinical trial
- PMID: 37753443
- PMCID: PMC10518503
- DOI: 10.1016/j.eclinm.2023.102142
Effect of unguided e-cigarette provision on uptake, use, and smoking cessation among adults who smoke in the USA: a naturalistic, randomised, controlled clinical trial
Abstract
Background: As summarised in the most recent Cochrane review, the few clinical trials on e-cigarettes are largely focused on smoking cessation. We aimed to determine the naturalistic uptake, use, and impact of e-cigarettes among adults who may or may not want to stop smoking.
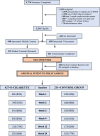
Methods: In this naturalistic, randomised, controlled clinical trial, adult smokers, across the motivational spectrum and with minimal history of e-cigarette use, were recruited online from the general community within 11 cities across the USA. Participants were randomly assigned (2:1) to either receive either a free 4-week supply of flavoured, tank-style e-cigarette, or not. E-cigarette group participants received a battery and device with up to 30 pre-filled tanks, offered among five flavours, with minimal instructions on use. The study's primary purpose was to descriptively assess naturalistic uptake and usage of the e-cigarette, and to secondarily assess its impact on smoking behavior. The latter, assessed through six months of follow-up, included: a) self-reported 7-day point prevalence abstinence, b) incidence of quit attempts, and c) smoking reduction. This trial is registered at ClinicalTrials.gov, NCT03453385.
Findings: Between 5/2018 and 3/2022, 638 adult smokers were enrolled and randomly assigned (427 in the e-cigarette group and 211 in the no-product control group). Uptake of e-cigarettes was robust: approximately 70% of participants used the product, with average usage exceeding 4 days per week during the initial 30 days. Based on an intent-to-treat approach where missing data is imputed as smoking, almost all behavioral outcomes favored the e-cigarette group relative to no-product control, including point prevalence abstinence at six months (Odds Ratio [OR] = 1.8; 95% Confidence Interval [CI] = 1.0-3.1), cumulative incidence of 24-hr quit attempts (OR = 1.5; 95% CI = 1.0-2.2), and having reduced smoking by at least 50% since baseline (OR = 1.8; 95% CI = 1.2-2.7). Results were similar under an alternative imputation.
Interpretation: Complementing cessation-focused trials, results suggest that unguided e-cigarette use also leads to smoking cessation, allaying the notion that causal effects of e-cigarettes on cessation are not reflective of real-world scenario of self-determined use. For smokers who may not be able to quit using existing pharmacologic approaches, e-cigarettes may be considered to achive that purpose.
Funding: National Cancer Institute.
Keywords: E-cigarettes; Public health; Randomised clinical trial; Smoking cessation.
© 2023 The Author(s).
Conflict of interest statement
KMC has served as a paid expert witness in litigation filed against the tobacco industry. KMG has provided consultation to Jazz Pharmaceuticals and has received research funding from Aelis Farma. MLG has served as a member of the Scientific Advisory Board to Johnson & Johnson; he has also consulted with both the World Health Organization and Campaign for Tobacco Free Kids on toxicity of tobacco products and tobacco control products; MLG is also a Member of the IASLC Tobacco Control and Smoking Cessation Committee; and a leadership role with the American Association for Cancer Research. JD is a co-owner of Behavioral Activation Tech LLC, a small business that develops digital interventions for behavioral health treatment. E-cigarette products (tanks and liquids) were purchased directly from NJoy; no study support provided from industry. All other authors declare no competing interests.
Figures


References
-
- Malas M., van der Tempel J., Schwartz R., et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016;18:1926–1936. - PubMed
-
- Chan G.C.K., Stjepanović D., Lim C., et al. A systematic review of randomized controlled trials and network meta-analysis of e-cigarettes for smoking cessation. Addict Behav. 2021;119 - PubMed
-
- Butler A.R., Lindson N., Fanshawe T.R., et al. Longer-term use of electronic cigarettes when provided as a stop smoking aid: systematic review with meta-analyses. Prev Med. 2022;165 - PubMed
-
- Hartmann-Boyce J., Lindson N., Butler A.R., et al. Cochrane database of systematic reviews. John Wiley & Sons; Oxford: 2022. Electronic cigarettes for smoking cessation.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

