Safety and efficacy of intravitreal anti vascular endothelial growth factor for severe posterior retinopathy of prematurity with flat fibrovascular proliferation
- PMID: 37753496
- PMCID: PMC10518743
- DOI: 10.5409/wjcp.v12.i4.220
Safety and efficacy of intravitreal anti vascular endothelial growth factor for severe posterior retinopathy of prematurity with flat fibrovascular proliferation
Abstract
Background: Intravitreal anti-vascular endothelial growth factor (IVA) injection is known to cause contraction of fibrovascular proliferation (FVP), when present in severe retinopathy of prematurity (ROP).
Aim: To assess the structural outcomes of IVA injection in the treatment of severe posterior ROP with significant FVP.
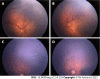
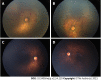
Methods: It was a retrospective study in which 36 eyes of 18 preterm babies who developed > 4 clock hours of FVP in zone I or posterior zone II, were treated with either intravitreal 0.625 mg bevacizumab or intravitreal 0.2 mg of ranibizumab. Favorable structural outcome included resolution of plus disease and FVP without the development of tractional retinal detachment. Secondary outcome measure included either full retinal maturation at follow-up or development of recurrent disease requiring additional treatment. Adverse outcomes included progression to retinal detachment.
Results: The mean gestational age of the 18 preterm babies was 30 wk (range 27-36), and mean birth weight was 1319 g (range 650-1980 g). Mean post-menstrual age (PMA) at the time of primary treatment was 35.5 wk (range 31-41 wk). All eyes showed regression of plus disease and FVP. 5 eyes of 3 babies showed reactivation of disease and were treated with repeat IVA (n = 2 eyes) or peripheral laser photocoagulation (n = 3 eyes) respectively. 16 out of 36 (44%) reached retinal vascular maturation at final follow up at 5 years.
Conclusion: There was good resolution of severe posterior ROP with FVP with IVA, with retinal maturity of 44% at 5 year follow-up and a reactivation rate of 13.8%. When the IVA injection is given prior to 37 wk PMA, while disease is in phase 2, it is less likely to cause contracture of pre-existing FVP.
Keywords: Anti-vascular endothelial growth factor injection; Contraction; Crunch phenomenon; Retinopathy of prematurity.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: We have no financial relationships to disclose.
Figures
References
-
- Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF, Li J, Liew M, Maier R, Zhu Q, Marlow N. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394:1551–1559. - PubMed
-
- Wu AL, Wu WC. Anti-VEGF for ROP and Pediatric Retinal Diseases. Asia Pac J Ophthalmol (Phila) 2018;7:145–151. - PubMed
LinkOut - more resources
Full Text Sources

