Sulawesi propolis induces higher apoptotic activity and lower inflammatory activity in a rat endometriosis model
- PMID: 37753513
- PMCID: PMC10518513
- DOI: 10.1016/j.eurox.2023.100204
Sulawesi propolis induces higher apoptotic activity and lower inflammatory activity in a rat endometriosis model
Abstract
Background: Endometriosis has a major impact on women's quality of life. The two primary pathologies are chronic inflammation and altered apoptotic activity. Sulawesi propolis has been shown to have known anti-inflammatory and pro-apoptotic properties in other diseases.
Objective: To investigate the effects of Sulawesi propolis in the rat endometriosis model.
Methods: An autologous endometriosis model was created in 60 female Wistar rats by laparotomy. Rats were divided into four groups (n = 15 in each group): control group (CG), dienogest group (DG), propolis 50 mg/kg body weight (BW)/day (P50) group, and propolis 100 mg/kg BW/day (P100) group. Each treatment group was divided into three different treatment durations (n = 5 in each treatment group): 2, 4 and 6 weeks. After treatment, laparotomy was performed to determine endometriotic tissue growth, apoptosis [caspase-3 and Bcl-2-associated X/Bcl-2 (Bax/Bcl)] and inflammation [prostaglandin-E2 (PGE2) and interleukin-1B (IL-1B)].
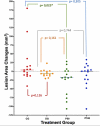
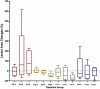
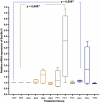
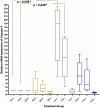
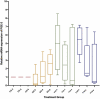
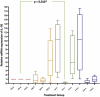
Results: A significant difference was seen in endometriotic tissue growth between the P50 group and the CG, with the greatest reduction in the P50 6-week (P50-6) group, reaching 70.66% of the initial area. Highest Bax/Bcl-2 mRNA expression was shown in the P50-4 and P100-4 groups, highest caspase-3 expression was shown in the P50-2 and P50-4 groups, and lowest IL-1B expression was shown in the P50-4 group; all differed significantly from the CG. No significant difference in PGE2S mRNA was found between the groups.
Conclusion: Sulawesi propolis extract suppressed endometriotic tissue growth in the rat model by increasing apoptotic activity. The effects were time-dependent, with 50 mg/kg BW as the optimal dose.
Keywords: Apoptotic; Chronic inflammation; Endometriosis; Propolis; Rats.
© 2023 The Authors.
Conflict of interest statement
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. H. Situmorang, A. Hestiantoro, S. Purbadi, P.E. Wuyung, R.A. Werdhani, A. Harahap, W. Permadi, M. Sahlan, W. Hadisaputra.
Figures



Similar articles
-
IN-SILICO dynamic analysis of Sulawesi propolis as anti-endometriosis drug: Interaction study with TNF alpha receptor, NF-kB, estrogen receptor, progesterone receptor and prostaglandin receptor.Ann Med Surg (Lond). 2021 Jun 17;67:102459. doi: 10.1016/j.amsu.2021.102459. eCollection 2021 Jul. Ann Med Surg (Lond). 2021. PMID: 34194730 Free PMC article.
-
Interleukin-6 expression on inflamed rat dental pulp tissue after capped with Trigona sp. propolis from south Sulawesi, Indonesia.Saudi J Biol Sci. 2017 Jul;24(5):1034-1037. doi: 10.1016/j.sjbs.2016.12.019. Epub 2016 Dec 28. Saudi J Biol Sci. 2017. PMID: 28663700 Free PMC article.
-
The effects of ulipristal on Bax/Bcl-2, cytochrome c, Ki-67 and cyclooxygenase-2 expression in a rat model with surgically induced endometriosis.Eur J Obstet Gynecol Reprod Biol. 2013 Jul;169(2):360-5. doi: 10.1016/j.ejogrb.2013.03.022. Epub 2013 Apr 22. Eur J Obstet Gynecol Reprod Biol. 2013. PMID: 23619346
-
Turkish Propolis and Its Nano Form Can Ameliorate the Side Effects of Cisplatin, Which Is a Widely Used Drug in the Treatment of Cancer.Plants (Basel). 2020 Aug 21;9(9):1075. doi: 10.3390/plants9091075. Plants (Basel). 2020. PMID: 32825574 Free PMC article.
-
Apoptosis and differential expression of apoptosis-related proteins in endometriotic glandular and stromal cells.J Soc Gynecol Investig. 2004 Jul;11(5):318-22. doi: 10.1016/j.jsgi.2004.02.005. J Soc Gynecol Investig. 2004. PMID: 15219886
References
-
- Sampson J.A. Heterotopic or misplaced endometrial tissue. Am J Obstet Gynecol. 1925;10:649–664.
-
- Vercellini P., Vigano P., Somigliana E., Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. - PubMed
-
- Gonzalez-Ramos R., Defrere S., Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98:520–528. - PubMed
-
- Figueiredo S.M., Nogueira-Machado J.A., Almeida B.M., et al. Immunomodulatory properties of green propolis. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:85–94. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

