Altered hepatic and intestinal homeostasis in a neonatal murine model of short-term total parenteral nutrition and antibiotics
- PMID: 37753583
- PMCID: PMC11901332
- DOI: 10.1152/ajpgi.00129.2023
Altered hepatic and intestinal homeostasis in a neonatal murine model of short-term total parenteral nutrition and antibiotics
Abstract
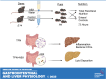
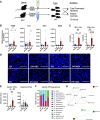
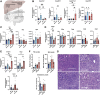
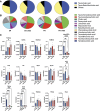
Parenteral nutrition (PN) prevents starvation and supports metabolic requirements intravenously when patients are unable to be fed enterally. Clinically, infants are frequently provided PN in intensive care settings along with exposure to antibiotics (ABX) to minimize infection during care. Unfortunately, neonates experience extremely high rates of hepatic complications. Adult rodent and piglet models of PN are well-established but neonatal models capable of leveraging the considerable transgenic potential of the mouse remain underdeveloped. Utilizing our newly established neonatal murine PN mouse model, we administered ABX or controlled drinking water to timed pregnant dams to disrupt the maternal microbiome. We randomized mouse pups to PN or sham surgery controls +/- ABX exposure. ABX or short-term PN decreased liver and brain organ weights, intestinal length, and mucosal architecture (vs. controls). PN significantly elevated evidence of hepatic proinflammatory markers, neutrophils and macrophage counts, bacterial colony-forming units, and evidence of cholestasis risk, which was blocked by ABX. However, ABX uniquely elevated metabolic regulatory genes resulting in accumulation of hepatocyte lipids, triglycerides, and elevated tauro-chenoxycholic acid (TCDCA) in serum. Within the gut, PN elevated the relative abundance of Akkermansia, Enterococcus, and Suterella with decreased Anaerostipes and Lactobacillus compared with controls, whereas ABX enriched Proteobacteria. We conclude that short-term PN elevates hepatic inflammatory stress and risk of cholestasis in early life. Although concurrent ABX exposure protects against hepatic immune activation during PN, the dual exposure modulates metabolism and may contribute toward early steatosis phenotype, sometimes observed in infants unable to wean from PN.NEW & NOTEWORTHY This study successfully established a translationally relevant, murine neonatal parenteral nutrition (PN) model. Short-term PN is sufficient to induce hepatitis-associated cholestasis in a neonatal murine model that can be used to understand disease in early life. The administration of antibiotics during PN protects animals from bacterial translocation and proinflammatory responses but induces unique metabolic shifts that may predispose the liver toward early steatosis.
Keywords: PNALD; liver; pediatrics; total parenteral nutrition.
Conflict of interest statement
J.F.P. is cofounder of Gateway Biome, Inc. There are no financial or intellectual conflicts of with this research and Gateway Biome, Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

